Elizabeth Brown browng@babe.net.au
An emulsion of sodium thiosulfate (called hypo by photographers) is used to stop development of exposed film. Thiosulfate converts undeveloped silver bromide grains in the film into water-soluble silver thiosulfate complexes that can be removed when the film is washed.
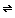 AgS2O3- + Br-
AgS2O3- + Br-S2O32-+ AgS2O3-
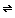 Ag(S2O3)3-
Ag(S2O3)3-
See these Web sites for more detail on the chemistry of photography:
-
Chemistry in Black and White Photography
| A laboratory exercise showing how photographic developers work. Includes a section on toning for iron, copper, and sepia. http://wwwchem.csustan.edu/chem1002/photog.htm (12/27/98) |
| The composition and preparation of film paper, and developing chemicals is described. http://www.freeyellow.com/members6/glsmyth/photomat.htm (3/19/99) |