Aspirin is made of a carboxylic acid called acetylsalicylic acid. It occurs in two forms in solution, depending on pH:
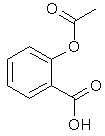
acetylsalicylic acid in acid solution
(fat soluble) |

acetylsalicylic acid in neutral and basic solution
(water soluble) |
Acetylsalicylic acid is in its fat-soluble form in stomach acid. It easily diffuses through the stomach
lining and into the cells underneath. Once there, it encounters a much higher pH and ionizes again (preventing it from diffusing back into the stomach). Aspirin suppresses the production of prostaglandins- hormones that
stimulate blood clotting, among other things. This causes capillaries in the stomach lining to leak. The amount of
bleeding is small for most people, but in some cases it can result in serious blood loss.
Keeping the acetylsalicylic acid in
ionic form or preventing it from dissolving until it reached the small intestine would prevent it from
causing bleeding.
Some manufacturers have tried to add
buffering agents to their tablets for this purpose. But the benefits of buffered aspirin are disputed.
Just about any buffering agent used in an antacid can be used in buffered aspirin. Bufferin, for example,
uses MgO. Other preparations use CaCO3.
Author: Fred Senese senese@antoine.frostburg.edu


