Let's use O2 as a specific example. It's known experimentally that the length and strength of the bond between the oxygens suggests a double bond. The valence bond theory views multiple bonds as overlaps between orbitals that lie off the bond axis, on top of an overlap that occurs on the bond axis. The overlap on the bond axis is called a sigma bond. The overlaps off the bond axis are called pi bonds.
- Draw a Lewis structure for the molecule. For O2, it's
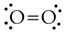
- Count how many electron 'regions' you have around each atom in the molecules. A region is either a bond or a lone pair. Multiple bonds count as just one region. In O2, there are 3 regions around each atom.
- Chose a hybridization scheme for each atom that is consistent with the number of regions around that atom:
Regions Possible
HybridizationShape 2 sp linear (w/ 2 unhybridized p's) 3 sp2 triangular planar (w/ 1 unhybridized p) 4 sp3 tetrahedral 5 dsp3 triangular bipyramidal 6 d2sp3 octahedral - Sketch the hybrid orbitals for each atom. For O2,

- Sketch the valence orbitals that are unhybridized. The O's are sp2 hybridized, so there is one unhybridized p orbital at right angles to the hybridized orbitals. The p orbital is drawn in blue:

- Overlap orbitals to get the right number of bonds. The oxygens have a hybridized sp2 orbital and an unhybridized p that can overlap to give the double bond. Notice that the hybridized orbital overlap is a sigma bond, because it is along the bond axis; the unhybridized p's overlap side to side to give a pi bond, which is off the bond axis.
