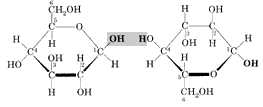
 Cellulose is a natural polymer, a long chain made by the linking of smaller molecules. The links in the cellulose chain
are a type of sugar: ß-D-glucose. Two unlinked molecules of ß-D-glucose are pictured at right [1].
The sugar units are linked when water is eliminated by combining the -OH group and H highlighted in gray. Linking just two of these sugars produces a disaccharide called cellobiose [2]. Cellulose is a polysaccharide produced by linking additional sugars in exactly the same way. The length of the chain varies greatly, from a few hundred sugar units in wood pulp to over 6000 for cotton.
Cellulose is a natural polymer, a long chain made by the linking of smaller molecules. The links in the cellulose chain
are a type of sugar: ß-D-glucose. Two unlinked molecules of ß-D-glucose are pictured at right [1].
The sugar units are linked when water is eliminated by combining the -OH group and H highlighted in gray. Linking just two of these sugars produces a disaccharide called cellobiose [2]. Cellulose is a polysaccharide produced by linking additional sugars in exactly the same way. The length of the chain varies greatly, from a few hundred sugar units in wood pulp to over 6000 for cotton.
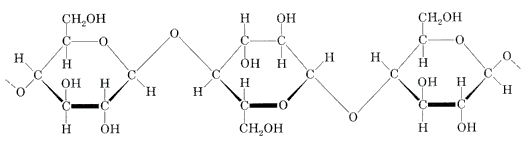
The cellulose chain bristles with polar -OH groups. These groups form many hydrogen bonds with OH groups on adjacent chains, bundling the chains together. The chains also pack regularly in places to form hard, stable crystalline regions that give the bundled chains even more stability and strength.
Cellulose is a major component of wood. Cellulose fibers in wood are bound in lignin, a complex polymer. Paper-making involves treating wood pulp with alkalis or bisulfites to disintegrate the lignin, and then pressing the pulp to matte the cellulose fibers together.
Cellulose is found in large amounts in nearly all plants, and is potentially a major food source. Unfortunately, human beings lack the enzymes necessary to cleave the linkages between the sugars in cellulose. In fact, crystallite cellulose is added to some foods to reduce the caloric value.
Notes
- Glucose is a six-carbon sugar that comes in two forms with different optical activity. The ß form, pictured above, has the hydroxide group on carbon 1 on the same face of the ring as the -CH2OH group. The
 form has the same hydroxide group on the opposite face from the -CH2OH group.
form has the same hydroxide group on the opposite face from the -CH2OH group.
- Sucrose, or table sugar, is the most familiar disaccaride, made by linking the sugars glucose and fructose in a different way.
References and bibliography
- C. Dorée, The Methods of Cellulose Chemistry, Chapman & Hall, London, 1947.
- The Merck Index, 8th ed., Merck & Co., Rahway NJ, 1968.