Jamelia
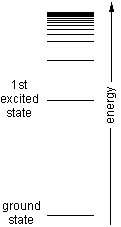 The energy ladder for a hydrogenlike atom is shown at left. There are three cases to consider:
The energy ladder for a hydrogenlike atom is shown at left. There are three cases to consider:
- If you're comparing two transitions with the same difference in quantum number, notice that the rungs on the bottom of the energy ladder are further apart than the rungs on top. That means that emission wavelengths are smaller for transitions low on the ladder.
- If you're comparing two transitions that involve the same quantum number, the one with the larger difference between quantum numbers will have the higher transition energy and the smaller wavelength.
- If you want to compare a pair of transitions that don't fit under (1) or (2), use the fact that the transition energies are proportional to (1/n22) - (1/n12), where n1 and n2 are the quantum numbers labelling the lower and upper states in the transition.