Search
Exam Guide
FAQ
Home Companion
Just Ask Antoine!
Periodic Table
Resources
Tutorials
Publisher
Project Antoine
Sponsor
Frostburg State University
Staff
F. A. Senese
Dept. of Chemistry
Methyl Orange
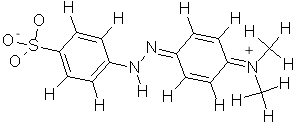
Here is the structure of methyl orange in acidic solution:
|
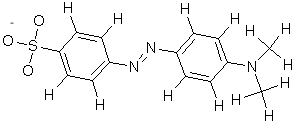
In the basic form of methyl orange, a hydrogen ion is lost from the -NN- bridge between the rings, and the electrons formerly used to bind the hydrogen neutralize the positive charge on the terminal nitrogen, so that it is no longer able to pi-bond. Solutions of the methyl orange appear yellow in alkaline solution.
|