Amy 9/9/2000
| Vocabulary | |
allotrope carbon combustion  element  oxidize  |
Carbon wasn't recognized as an element until the seventeenth century, after Robert Boyle suggested that an element was a substance that could not be decomposed into simpler substances. Antoine Lavoisier's pioneering chemistry textbook Traité Élémentaire de Chimie, published in Paris in 1789, lists carbon as an "oxidizable and acidifiable nonmetallic element" [3].
Carbon occurs naturally in several forms. Diamond, graphite, and amorphous carbon have been known throughout written history, but it was not known that they were different forms of the same substance until the late eighteenth century. If alchemists had directed their efforts towards conversion of graphite to diamonds instead of futilely trying to convert lead into gold, the early history of chemistry would have been quite different!
Antoine Lavoisier showed that diamonds are a form of carbon in 1772. He burned carefully weighed diamond and carbon samples and showed that both substances produced no water vapor and the same amount of carbon dioxide gas per gram. Graphite was thought to be a form of lead until 1779, when Carl Wilhelm Scheele showed that graphite produces the same amount of carbon dioxide gas per gram as amorphous carbon does.
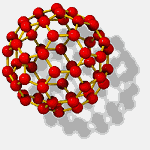 A new form of carbon
was discovered in 1985 by
Harold Kroto (Sussex University), Robert Curl, Jr. (Rice University), and Richard Smalley (Rice University).
The new form was called "buckministerfullerene" because its molecules resemble the geodesic domes designed by architect Buckminister Fuller for the 1967 World's fair. "Buckyballs" are being considered in the design of next-generation lubricants, drug delivery systems, industrial catalysts, and nanoscale machinery.
A new form of carbon
was discovered in 1985 by
Harold Kroto (Sussex University), Robert Curl, Jr. (Rice University), and Richard Smalley (Rice University).
The new form was called "buckministerfullerene" because its molecules resemble the geodesic domes designed by architect Buckminister Fuller for the 1967 World's fair. "Buckyballs" are being considered in the design of next-generation lubricants, drug delivery systems, industrial catalysts, and nanoscale machinery.
References and notes
- Carbon, from Elementymology & Elements Multidict by Peter van der Krogt.
- D. P. Simpson, Cassell's Latin Dictionary, Macmillan, New York (1959).
- Antoine Lavoisier's original table of elements, taken from theTraité Élémentaire de Chimie, 1789, as quoted in J. R. Partington's A Short History of Chemistry (Dover, 1989, ISBN 0486659771). The preface to Lavoisier's text is available in HTML at Carmen Giunta's Classical Chemistry site. Giunta has also provided an English translation of Lavoisier's table.