CO2 must first cross the surface of the liquid:
CO2(g) 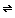 CO2(aq)
CO2(aq)
Waters first hydrogen-bond to the oxygen atoms on the ends of the CO2 and additional The CO2 rather slowly acquires a shell of water molecules. A fraction of these hydrated carbon dioxide molecules react with the water to produce carbonic acid (H2CO3):
CO2(aq) + H2O 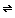 H2CO3(aq)
H2CO3(aq)
The activation energy for the reaction is rather high, since it involves bending a stable, linear CO2 molecule (with a water parked oxygen-down over the carbon) into a Y-shaped O=C(OH)2 molecule. As a result, the reaction proceeds very slowly.