What is the structure of nitrous oxide?

| Linear structure of N2O. Click on the picture for a 3D Chime model. |
Formal charge considerations suggest that the most important resonance structures are
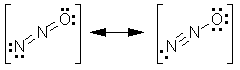 A third structure involving a triple bond between the nitrogen and oxygen is unlikely because that would result in high formal charges. These resonance structures can be used to explain experimental bond lengths. The nitrogen-nitrogen bond is 1.126 Angstroms long
which is slightly longer than the triple bond length in N2 (1.098 Angstroms).
The nitrogen-oxygen bond is 1.186 Angstroms long. This is longer than the typical N=O bond (about 1.14 Angstroms), which agrees with the prediction of partial single-bond character for the NO bond in N2O.
A third structure involving a triple bond between the nitrogen and oxygen is unlikely because that would result in high formal charges. These resonance structures can be used to explain experimental bond lengths. The nitrogen-nitrogen bond is 1.126 Angstroms long
which is slightly longer than the triple bond length in N2 (1.098 Angstroms).
The nitrogen-oxygen bond is 1.186 Angstroms long. This is longer than the typical N=O bond (about 1.14 Angstroms), which agrees with the prediction of partial single-bond character for the NO bond in N2O.
The molecule is not strongly polar, despite the large electronegativity difference between nitrogen and oxygen. The resonance structures again can be used to explain why: the negative formal charge is concentrated on the terminal nitrogen in the structure at left,. The oxygen bears a negative formal charge in the other structure. Each structure is polar, but the dipole moments point in opposite directions. The dipole moment is expected to be small due to cancellation of the contributions from both structures.
The low polarity of the gas makes it both fat and water soluble. This allows it to travel through the bloodstream and into the fatty membranes of nerve cells where it produces its characteristic effects. Nitrous oxide's fat solubility and low toxicity make it an ideal propellant for whipped cream. It dissolves easily in cream under pressure and bubbles out of solution when the pressure is released, creating a fine creamy foam.
How was nitrous oxide discovered?
Joseph Priestley (the discover of oxygen, soda pop, and carbon dioxide) described the preparation of "nitrous air diminished" in his classic 1772 paper Observations on Different Kinds of Air. The gas he collected over mercury supported combustion, but did not itself burn. He described the bizarre enlarged double-cone of a candle flame in nitrous oxide with great excitement: "I have now discovered an air five or six times as good as common air... nothing I ever did has surprised me more, or is more satisfactory." But Priestley seems to have overlooked the powerful psychoactive effects of breathing nitrous oxide.Over 20 years later, Humphry Davy wrote about the intoxicating effects of nitrous oxide, comparing them to the effects of alcohol. Breathing air after inhaling high concentrations of the gas sometimes lead to hysterical laughter. It also lead to a cessation of pain and "laughing gas" became the first artifical anaesthetic. It was in common use by surgeons in the late 19th century, and is still widely used in dentistry today.
Why is nitrous oxide used in rocket fuels and racing cars?
Nitrous oxide supports combustion better than air does. The N2O molecule dissociates at temperatures well below what is required for combustion, delivering an atom of oxygen and freeing molecular nitrogen:
N2O(g)  N2(g) + O(g)
N2(g) + O(g)
If there is a large excess of nitrous oxide in the engine, the fuel will detonate. At the extremely high temperature produced by the explosion, oxygen atoms freed by decomposing N2O will attack the engine metal, severely damaging it.
How is nitrous oxide prepared?
The gas is present in trace amounts in Earth's atmosphere as a result of high temperature reactions between nitrogen and oxygen.Industrially the gas is prepared by gently heating ammonium nitrate [Archibald]:
NH4NO3(s)  2 H2O(g) + N2O(g)
2 H2O(g) + N2O(g)
Is breathing nitrous oxide dangerous?
While anaesthetists mix nitrous oxide with oxygen, recreational users sometimes do not. Breathing gas rushing from a cylinder can quickly flush all air out of the lungs and cause suffocation.Another hazard of nitrous oxide stems from the fact that it is NOT an ideal gas. N2O molecules attract each other. The attractions require energy to break, so the expansion absorbs heat and the temperature of the gas plummets. A rapid expansion of nitrous oxide can cool it enough to cause frostbite. People doing whippets have actually frozen their lips, tongues, or vocal cords- and under the anaesthetic influence of nitrous oxide, the damage is done before any pain is felt.
Nitrous oxide has also been linked to birth defects, nerve damage, and permanent organ damage.
References and Links
- A Short History of Chemistry, J. R. Partington, Dover, New York, 1989.
- Engravings showing the apparatus Priestley used to isolate nitrous oxide can be found in Partington's book, along with much interesting biographical information.
- The Preparation of Pure Inorganic Substances, E. H. Archibald, Wiley, New York, 1932.
- Preparation of nitrous oxide by thermal decomposition of ammonium nitrate.
| Science teacher Steve Silverman's wry take on the discoverer of oxygen, who (quite incidentally) is also the discoverer of laughing gas, soda pop, rubber erasers, and carbon dioxide. Priestley's story is filed under Useless Information, along with tales about vinegar as a hazardous substance, forgotten geniuses, how the fortunes of war affected the naming of aspirin, and the horrific dangers of playing with 19th century matches. http://home.nycap.rr.com/useless/priestly/priestly.html (8/14/98) |