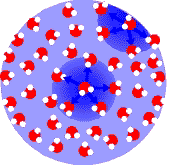
 Water molecules attract each other rather strongly due to their polarity. Molecules deep within the drop are pulled every which way by their neighbors. Since they are surrounded more or less symmetrically, they experience little or no net force.
Water molecules attract each other rather strongly due to their polarity. Molecules deep within the drop are pulled every which way by their neighbors. Since they are surrounded more or less symmetrically, they experience little or no net force.
Molecules on the droplet surface don't have neighbors on one side. There is a strong net force from the tug of the water molecules below them, which pulls them towards the center of the droplet.
Surface tension is the work you have to do to increase the area of the drop by a unit amount. Since work has units of force times length, surface tension has units of force per unit length.
A small amount of liquid in a very thin dropper won't run out unless you blow it out- it's held in the tube by the surface tension of the drop on the tip, which exerts an upward force. If there is enough liquid in the tube, though, there will come a point where the weight of the liquid overwhelms upward force, and the drop will fall.
If surface tension is force per unit length, you can crudely predict the weight of that droplet by multiplying the circumference of the dropper tip by the liquid's surface tension. For example,
- a 1 mm diameter dropper has a circumference of pi times the diameter, or 0.314 cm.
- water has a surface tension of about 72.9 g/s2. These are units of force (g cm/s2) per length (cm).
- The upward force on due to surface tension is then about 0.314 cm × (72.9 g/s2) = 23 g cm/s2.
- The weight required to overcome the upward force will also be 23 g cm/s. That corresponds to a droplet mass of about 0.023 g.