Shan GizmoRichter@worldnet.att.net
The stronger the forces that act between molecules of a substance, the higher the melting point tends to be.
Melting point trends for the first four periods are shown in the diagram below.
The trends are very complex because many different factors influence the forces between atoms (or molecules) in an element.
Notice that for each period beyond the first, the melting point rises to a maximum somewhere around the middle of the period and then falls off to a minimum value at the end of the period:
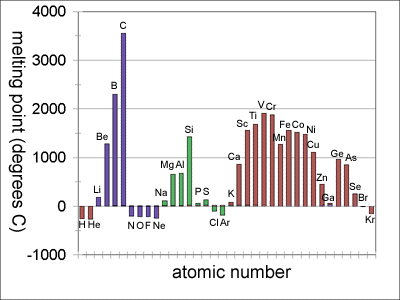
The melting points of the first period elements are extremely low, because forces between H2 molecules and between helium atoms are exceptionally weak.
In the second period, there is a gradual transition from relatively weak metallic bonding in lithium to strong network covalent bonding in carbon. Nitrogen, oxygen, and fluorine also form strong covalent bonds but they can't form networks of bonds the way carbon does. Atoms of these elements pair up to form diatomic molecules. While the attractive forces within atoms in the diatomic gas molecules is strong, the forces between molecules is very weak. That causes the sharp dropoff in melting point after carbon in the second period.
The trend is repeated in a more subdued way in the third period. There is a jump in melting point from aluminum to silicon where bonding changes from primarily metallic to more covalent. P and S are better able to link into chains and rings than their second period counterparts, and have much higher melting points than N2 and O2. In the fourth period, the rise and fall of melting points across the period is even more muted. Note the discontinuity going from gallium (Ga) to germanium (Ge) at the metal/metalloid border.