Adam 6/17/05
- Solid forms: Ca+2(aq) + 2 OH-(aq)
 Ca(OH)2(s)
Ca(OH)2(s)
- Solid dissolves: Ca(OH)2(s)
 Ca+2(aq) + 2 OH-(aq)
Ca+2(aq) + 2 OH-(aq)
If you put solid Ca(OH)2 into water, both processes run at once. Eventually, a balance is struck between the two, and solid forms at exactly the same rate that it dissolves. There will be no apparent change in the amount of solid or in the concentrations of the calcium and hydroxide ions in the tank at that point. Equilibrium has been established.
Dumping more hydroxide ions into the solution will upset that equilibrium. More hydroxide ions mean more encounters between between calcium and hydroxide ions in solution, so more solid calcium hydroxide will form in the first process. The extra hydroxide won't directly affect the second process; it runs at the same rate as before. The net result is that some of the calcium hydroxide precipitates.
All of this assumes that the solution in the tank was saturated with calcium hydroxide before the sodium hydroxide was added. What if it wasn't? You'll need to do an equilibrium calculation based on the reaction
Ca(OH)2(s) 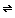 Ca+2(aq) + 2OH-(aq)
Ca+2(aq) + 2OH-(aq)
- Set up the equilibrium law for the reaction. Remembering that solid reactants and products are not written in equilibrium laws, we have
Ksp = [Ca+2][OH-]2
where Ksp is the solubility product constant (about 6.5 × 10-6 for Ca(OH)2) and [Ca+2] and [OH-] represent the equilibrium concentrations of calcium and hydroxide ions. The expression on the right side of equation is called the solubility product or ion product. - Calculate the ion product from the right hand side of the equilibrium law, using the actual concentrations of Ca+2 and OH- in the tank. For example, if the solution in the tank were 0.010 M Ca(OH)2 and 0.001 M NaOH, we have:
concentration of Ca+2 = 0.010 M concentration of OH- = 2 x 0.010 M + 0.001 M = 0.021 M ion product = (0.010)(0.021)2 = 4.4 × 10-6 - Compare the ion product to Ksp. If the ion product is greater than the Ksp, the solution is supersaturated, and precipitate will form. If the ion product is less than or equal to the Ksp, no precipitate will form. For example, the ion product we calculated in the previous step is less than Ksp (4.4 × 10-6 < 6.5 × 10-6), so we wouldn't expect any precipitate to form in the tank.
Your experiment illustrates the common ion effect: forcing an ionic compound to precipitate from solution by adding one of its ions to the solution. It's also called "salting out" a precipitate. It has a number of important applications. For example, you can use this trick to remove heavy metal ions from wastewater. You can also use it to understand why the body can't absorb minerals present in some foods; if an ion such as iron or calcium is "salted out" in the digestive system, the body won't be able to absorb it very efficiently.
References and Notes
- An interactive solution to a similar problem can be found here, on Wiley's Student Companion Site for Chemistry: Matter and Its Changes, 4th Ed.
