
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
| Everyday chemistry |
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Chemistry of everyday life Chemistry of everyday life | Print | Comment |
| Previous Question | Next Question |
How is coffee decaffeinated?
-
-
Caffeine is a mild stimulant that may cause nervousness, irritability, insomnia, and over-the-top website development. A 7 ounce cup of coffee can contain anywhere from 30 to 180 milligrams of caffeine- a little jolt that some coffee drinkers would rather do without. Caffeine has a bitter taste, so removing coffee's buzz need not spoil its flavor. Several technologies for selectively extracting caffeine have been used over the last century.
Vocabulary benzene
caffeine
chloroform
critical point
critical pressure
critical temperature
dichloromethane
distillation
extraction
recrystallization
reverse osmosis
supercritical fluid
Organic solvent extraction
Toxic solvents were used in early decaffeination efforts, including benzene, chloroform, and trichloroethylene (TCE). Dichloromethane, CH2Cl2, became the solvent of choice in the early 1970's because of its lower toxicity and its ability to selectively dissolve caffeine without carrying off sugars, peptides, and flavor ingredients. However, when evidence suggested that CH2Cl2 might be carcinogenic, its use was sharply curtailed [1].Ethyl acetate was used as a replacement for dichloromethane during the 80's and early 90's. Although it is moderately toxic, coffee makers touted ethyl acetate as "natural" because it was present in fruit.
Two nontoxic and more environmentally benign solvents are now used: water, and supercritical fluid carbon dioxide.
Water extraction
Hot water extracts both flavor ingredients and caffeine from green coffee beans. If the extract is passed through activated charcoal, most of the caffeine is removed. Soaking the original beans in the decaffeinated extract then restores most of their flavor.In the Swiss water process, caffeine-free "flavor charged water" is used to extract the caffeine from green coffee beans. Since the flavor charged water is already saturated with flavor ingredients, only caffeine moves from the beans to the water.
Supercritical fluid CO2 extraction
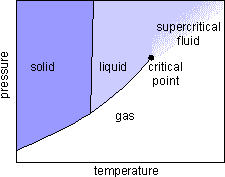 When a sealed vial containing both gaseous and liquid carbon dioxide under high pressure is heated, the liquid density drops while the gas density rises. If the pressure is above 72.8 atm, and the temperature rises above
304.2 K, the density of the liquid and the density of the gas become identical.
The meniscus between the liquid and gas phases vanishes. The carbon dioxide becomes a supercritical fluid
which has both gaslike and liquidlike properties. The fluid fills the container like a gas, but can dissolve substances like a liquid. Supercritical fluid carbon dioxide is an excellent nonpolar solvent for many organic compounds, including caffeine.
When a sealed vial containing both gaseous and liquid carbon dioxide under high pressure is heated, the liquid density drops while the gas density rises. If the pressure is above 72.8 atm, and the temperature rises above
304.2 K, the density of the liquid and the density of the gas become identical.
The meniscus between the liquid and gas phases vanishes. The carbon dioxide becomes a supercritical fluid
which has both gaslike and liquidlike properties. The fluid fills the container like a gas, but can dissolve substances like a liquid. Supercritical fluid carbon dioxide is an excellent nonpolar solvent for many organic compounds, including caffeine.
The extraction process is simple. Supercritical carbon dioxide is forced through green coffee beans. Its gaslike behavior allows it to penetrate deep into the beans, and it dissolves 97-99% of the caffeine present.
Coffee manufacturers recover the caffeine and resell it for use in soft drinks and medicines. The caffeine-laden CO2 is sprayed with high pressure water and caffeine is then isolated by a variety of methods, including charcoal adsorption, distillation, recrystallization, or reverse osmosis.
Genetic engineering
Caffeine extraction is expensive, and some of coffee's more fragile flavor ingredients are always lost or changed in the extraction process. Recent advances in biotechnology may make caffeine extraction obsolete.The final steps of caffeine synthesis in tea and coffee plants are catalyzed by an enzyme called caffeine synthase. Researchers in Japan and Scotland reported the first successful cloning of the gene that codes for caffeine synthase in 2000 [3]. Subsequent investigations will probably reveal ways to inactivate the gene, leading to tea and coffee plants that are unable to produce caffeine.
References
-
1. "Coffee Decaffeination Process and Cancer", in Cancer Facts, National Cancer Institute, National Institutes of Health, http://cis.nci.nih.gov/fact/pdfdraft/3_risk/fs3_16.pdf.
- 2. O'Brien, M.J., Spence, J.E., Skiff, R.H., Vogel, G. J., Prasad, R.: "Caffeine Recovery from Supercritical Carbon Dioxide", US Patent 4.996.317, 1991
- 3. "Plant biotechnology: Caffeine synthase gene from tea leaves", Misako Kato, Kouichi Mizuno, Alan Crozier, Tatsuhito Fujimura, Hiroshi Ashihara, Nature 406, 956 - 957 (31 August 2000).
Decaffeinating Coffee
(Scientific American) 
A brief article by Saul Katz describes various methods for decaffeinating coffee. The article includes a step-by-step illustration of supercritical fluid carbon dioxide extraction of caffeine.
http://www.sciam.com/0697issue/0697working.html (04/06/99) - 2. O'Brien, M.J., Spence, J.E., Skiff, R.H., Vogel, G. J., Prasad, R.: "Caffeine Recovery from Supercritical Carbon Dioxide", US Patent 4.996.317, 1991
Supercritical fluid resources
-
Introduction to Supercritical Fluids
(Phasex Corporation)
Supercritical Research Group
A brief introduction to supercritical CO2 extraction, an economically important technology used to decaffeinate coffee and tea, extract hops flavors in brewing, and extracting aromas and flavors from spices and herbs.
http://www.phasex4scf.com/scf.htm (7/2/99)(University of South Wales) 
An introduction to supercritical fluids and their applications, including decaffeination of coffee and waste treatment.
http://www.ceic.unsw.edu.au/centers/SF1/scf.htm (04/06/99)Coffee chemistry resources
-
Jamaican Coffee
(Robert Lancashire, UWI, Mona, Jamaica)
Key Facts about Decaffeination
Robert Lancashire shows that there is more than caffeine behind the chemistry of coffee. Chime molecular models, gas chromatographs, and mass spectra for the major chemical components and flavor ingredients.
http://wwwchem.uwimona.edu.jm:1104/lectures/coffee.html (3/27/99)(Choice Coffee)
The Chemistry of Things Jamaican
Details about various methods for decaffeination using methylene chloride, ethyl acetate, activated charcoal, triglycerides, and supercritical carbon dioxide.
http://www.worldwidemart.com/choice/decaf.html (12/22/99)(Robert Lancashire, University of the West Indies at Mona, Jamaica) 
Robert Lancashire's The Chemistry of Things Jamaican reveals the essential ingredients in tropical products. Created over the last four years, the site is a treasure trove that includes the chemistry and natural history of spices, Jamaican coffee, Carribean fruits and vegetables, Jamaican rum, and more. The pages are illustrated with many striking images and interactive Chime molecular models. Dr. Lancashire's pioneering work on interactive J-CAMP GC/MS spectra can also be found on the site.
http://wwwchem.uwimona.edu.jm:1104/chemlect.html (7/22/98) -
1. "Coffee Decaffeination Process and Cancer", in Cancer Facts, National Cancer Institute, National Institutes of Health, http://cis.nci.nih.gov/fact/pdfdraft/3_risk/fs3_16.pdf.
General Chemistry Online! How is coffee decaffeinated?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/consumer/faq/decaffeinating-coffee.shtml