
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
| Everyday chemistry |
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Chemistry of everyday life Chemistry of everyday life | Print | Comment |
| Previous Question | Next Question |
What are triclocarban and triclosan (ingredients in some antiseptic soaps)?
- I've noticed that a chemical called "triclocarban" is in many soaps. It's said to kill many types of bacteria in very small amounts.What is the formula for triclocarban and how does it kill that many different bacteria?
Barf 6/27/99 -
Triclocarban (also called TCC, Cutisan, Solubacter, and trichlorocarbanilide) is a trivial name for 3-(4-chlorophenyl)- 1-(3,4-dichlorphenyl)urea:
Vocabulary active site 
enzyme
fatty acid
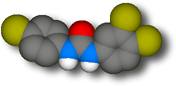
Triclocarban. Click on the structures for a 3D Chime molecular model.
Triclosan (also known as CH 3635, Irgasan Ch 3635, Irgasan DP 300, and Ster-Zac) is shown at left; it has a similar structure.
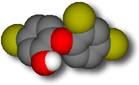
Triclosan. Click on the structures for a 3D Chime molecular model. Triclosan and triclocarban have been used as effective antiseptics [1] in soap since the 1960's. Triclosan has been incorporated into a wide range of consumer goods, including cosmetics, toothpaste, and plastics for children's toys and kitchen and table utensils.
Neither substance is very soluble in water, but both are fat-soluble and easily cross cell membranes. Once inside the cell, triclosan poisons a specific enzyme that many bacteria and funguses need for survival [2,3]. Triclosan blocks the active site of an enzyme called enoyl-acyl carrier-protein reductase (ENR for short), preventing the bacteria from manufacturing fatty acids it needs for building cell membranes and other vital functions. Humans don't have this enzyme, so triclosan is harmless to them. One molecule of triclosan permanently disables an ENR molecule, which explains why triclosan has powerful antibiotic action even at very low concentrations. Triclocarban's structural similiarity suggests a similiar mode of action.
The highly specific way that triclosan kills has researchers concerned about its role in fostering antibiotic-resistant strains of bacteria [2]. Researchers have recently demonstrated that mutations in the bacterial gene that produces ENR can produce triclosan-resistant bacteria. Because triclosan is now so widespread in the environment, it's likely that new antibiotics targeting ENR would be ineffective.
References
- Yackovich, F., N. K. Poulsen, and J. E. Heinze, "Validation of the Agar Patch Test Using Soap Bars which Deposit Different Amounts of Triclocarban", J. Soc. Cosmet. Chem., 37: 99-104 (1986).
- McMurray, L. M., Oethinger, M, Levy, S. B., "Triclosan targets lipid synthesis", Nature 394, 531-32 (1998). Abstract
- Levy, C. W., Roujeinikovai, A., Sedelnikova, S., Baker, P. J., Stuitje, A. R., Slabas, A. R., Rice, D., & Rafferty, J. B., "Molecular Basis of Triclosan Activity", Nature, 398, 383-384 (1999). Abstract
- Triclocarban, National Toxicology Program Chemical Repository Data Sheet (Radian Corporation, August 29, 1991), National Institute of Health, USA. WWW: http://ntp-server.niehs.nih.gov/.
- Triclocarban, BIAM (Automated Drug Databank), WWW: http://www.biam2.org/www/Sub3342.html
Related Web Links
Soaps and Detergents(Soap & Detergent Association) 
A general explanation of the detergent terminology, including a table showing the function of each ingredient in common cleaning products. An illustrated introduction to soap chemistry is included elsewhere on the site.
http://www.sdahq.org/sdalatest/html/soapproducts1.htm (6/27/99)
General Chemistry Online! What are triclocarban and triclosan (ingredients in some antiseptic soaps)?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/consumer/faq/triclosan.shtml