
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Companion Notes
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
| The periodic table |
Home  Companion Notes Companion Notes | Print | Comment |

|
Learning objectives
- Understand the rationale behind the periodic table
 ; view the table as an ordered database of element properties.
; view the table as an ordered database of element properties.
- Explain how the periodic table reflects the quantum mechanical structure of the atom.
- Explain and use periodic trends
 in:
in:
- Explain the connection between ionization energy and metallic character.
Before you start...
- Review the rationale behind quantum numbers.
- Be able to list sets of allowable quantum numbers for a given shell.
- Understand the concept of orbitals.
- Be able to explain how orbitals are grouped into subshells and shells.
- Be able to write electron configurations
 for atoms.
for atoms.
Lecture outline
The link between material properties and microscopic structure is a central theme in chemistry. These lectures use the periodic table to explore the connections between the properties of the elements and their electron configurations.
Quantum numbers and the periodic table
- An element's location on the periodic table reflects the quantum numbers of the last orbital filled
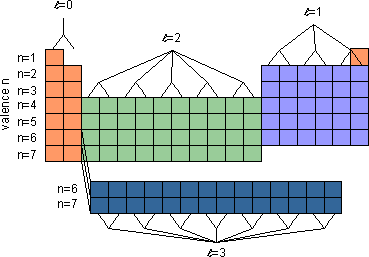
- The period
 indicates the value of principal quantum number
indicates the value of principal quantum number for the valence shell
for the valence shell
- the lanthanides
 and actinides
and actinides are in periods 6 and 7, respectively.
are in periods 6 and 7, respectively.
- the lanthanides
- The block
 indicates value of azimuthal quantum number
indicates value of azimuthal quantum number (
( ) for the last subshell
) for the last subshell that received electrons in building up the electron configuration.
that received electrons in building up the electron configuration.
- blocks are named for subshells (s, p, d, f)
- Each block contains a number of columns equal to the number of electrons that can occupy that subshell
- The s-block (in orange) has 2 columns, because a maximum of 2 electrons can occupy the single orbital in an s-subshell.
- The p-block (in purple) has 6 columns, because a maximum of 6 electrons can occupy the three orbitals in a p-subshell.
- The d-block (in green) has 10 columns, because a maximum of 10 electrons can occupy the five orbitals in a d-subshell.
- The f-block (in dark blue) has 14 columns, because a maximum of 14 electrons can occupy the seven orbitals in a f-subshell.
- questions to ponder
- What would the periodic table look like in a hypothetical universe where:
- there were 3 possible values of ms, instead of 2?
- the angular momentum quantum number could take on values from 1 to n-1 only?
- values of m
 = 0 were not allowed?
= 0 were not allowed?
- the maximum value of n were 5?
- What would the periodic table look like in a hypothetical universe where:
Factors affecting the valence shell
| Factors (in order of decreasing importance) | Effect | |
| 1. | valence principal quantum number n | Larger n means a larger valence shell (because n controls the size of orbitals) |
| 2. | nuclear charge Z | Larger Z means a smaller valence shell (because higher positive charge on the nucleus attracts the valence electrons, and pulls them inward) |
| 3. | number of core electrons | More core electrons means a larger valence shell (because highly penetrating core electrons repel valence electrons, and push them farther from the nucleus) |
Atomic radius
- what does atomic radius really mean?
- atoms have no definite surface
- a simple model: bound atoms are like touching spheres
- adding atomic radii for two bound atoms gives an estimate of bond length

Explaining periodic trends in atomic radius. See the section on factors affecting the valence shell above.
trend valence
nZ # core
electronsnet effect on atomic radius going right across main group rows... no change increases no change the increase in Z causes a decrease in radius going right across transition series... no change increases increases the increase in Z causes a decrease in radius, but the increase in the number of core electrons causes an increase. The two competing effects cause a small decrease, then small increase! going down groups... increases increases increases three competing effects; but n is strongest, so radius increases. - using the trends
- to compare atoms in different groups and different
periods, look for atoms that must be intermediate in size
- this isn't always possible!
- example: Which is larger, a silicon atom, or a selenium atom?
- to compare atoms in different groups and different
periods, look for atoms that must be intermediate in size
Ionic radius
- periodic trends parallel those of atomic radius
- cations are always smaller than the parent atom
- removing an electron decreases electron-electron repulsion, so the electron clouds contract
- emptying the valence shell completely leaves only electrons with lower n value
- anions are always larger than the parent atom
- adding an electron to an atom increases electron-electron repulsion and swells the electron cloud
- comparing radii for isoelectronic
 ions and atoms
ions and atoms
- size within isoelectronic series is affected only by Z
- example
F-, Ne, and Na+ are isoelectronic, with Z = 9, 10, and 11, respectively. All have identical valence n and identical numbers of electrons, so the larger Z is, the smaller the atom or ion. Na+ is the smallest and F- is the largest.
Ionization energy
- ionization energy is the minimum amount of energy required to remove an electron from an atom or ion in the gas phase
- normally, ionization removes valence electrons first
- valence electrons are farthest from nucleus on average, so they feel the least attraction for the nucleus and are easiest to remove
- end of valence electrons is marked by a big jump in ionization energies
Na(g)  Na+(g) + e-
Na+(g) + e- H = +496 kJ
H = +496 kJfirst ionization energy Na+(g)  Na+2(g) + e-
Na+2(g) + e- H = +4560 kJ
H = +4560 kJsecond ionization energy - core orbitals have lower n, and are much more penetrating than valence orbitals
- proximity to nucleus makes core electrons much more difficult to remove
- core noble gas configurations have high stability
- factors affecting ionization energy
- atomic radius
- smaller atoms hang on to valence electrons more tightly, and so have higher ionization energy
- charge
- the higher the positive charge becomes, the harder it is to pull away additional electrons
- second ionization energy is always higher than the first
- orbital penetration
- It's easier to remove electrons from p orbitals than from s orbitals
- electron pairing
- within a subshell, paired electrons are easier to remove than unpaired ones
- reason: repulsion between electrons in the same orbital is higher than repulsion between electrons in different orbitals
- example
On the basis of gross periodic trends, one might expect O to have a higher ionization energy than N. However, the ionization energy of N is 1402 kJ/mol and the ionization energy of O is only 1314 kJ/mol. Explain.
Taking away an electron from O is much easier, because the O contains a paired electron in its valence shell which is repelled by its partner.
- atomic radius
Why metals are metals
- the ionization energy of metallic elements is very low
- valence electrons are easily lost, and shared among all atoms in the metal
- this 'sea' of valence electrons binds together the metal cations and gives metals their characteristic properties
- mobility of electrons in the sea explains metal's ability to conduct electricity and heat
- metals are workable because cations can slide past each other but still be bound by the electron sea
- comparing metals
- more valence electrons means stronger metal
- higher positive charge on cations, higher negative charge on sea = stronger bonding
Explaining elemental properties: the s block elements
| property of alkali metals | explanation |
metallic |
very low ionization energy; the electron sea model works well for alkali metals |
soft |
ns1 valence configuration contributes just 1 electron to the electron sea. The sea is weak. Metal cations aren't tightly bound and it's easy to slide them past each other. |
low densities |
Alkali metals have the largest radii and lowest atomic weight in each period. Low mass in high volume = low density. |
highly reactive |
very low ionization energies make alkali metals good electron donors in redox reactions. |
- the alkaline earth metals (Group IIA)
- soft, but harder than alkali metals
- ns2 valence configuration = more electrons in the sea = more tightly bound metal cations
- reactive, but not as reactive as alkali metals
- ionization energies are not as low as alkali metals
- salts less soluble than those of the alkali metals
- higher cation charge concentrated on smaller cations makes it hard to pull apart ionic lattices
- soft, but harder than alkali metals
newsletter describing updates,
new features, and changes
on this site.
General Chemistry Online! The periodic table
Copyright © 1997-2005 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/periodic/index.shtml