
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
| Acids & bases |
Reaction rates
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Acids and bases Acids and bases | Print | Comment |
| Previous Question | Next Question |
What is the buffer system in buffered aspirin?
- I tested the pH of six different types of Aspirin. They are: the generic brand, children's aspirin, regular strength, buffered aspirin, and aspirin that I made. The pH of the Buffered aspirin was a lot higher than the others. What is the buffer system in buffered aspirin?
Shaun McCloskey -
Aspirin is made of a carboxylic acid called acetylsalicylic acid. It occurs in two forms in solution, depending on pH:
Vocabulary carboxylic acid 
pH
Acetylsalicylic acid is in its fat-soluble form in stomach acid. It easily diffuses through the stomach lining and into the cells underneath. Once there, it encounters a much higher pH and ionizes again (preventing it from diffusing back into the stomach). Aspirin suppresses the production of prostaglandins- hormones that stimulate blood clotting, among other things. This causes capillaries in the stomach lining to leak. The amount of bleeding is small for most people, but in some cases it can result in serious blood loss.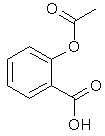
acetylsalicylic acid in acid solution
(fat soluble)
acetylsalicylic acid in neutral and basic solution
(water soluble)Keeping the acetylsalicylic acid in ionic form or preventing it from dissolving until it reached the small intestine would prevent it from causing bleeding. Some manufacturers have tried to add buffering agents to their tablets for this purpose. But the benefits of buffered aspirin are disputed.
Just about any buffering agent used in an antacid can be used in buffered aspirin. Bufferin, for example, uses MgO. Other preparations use CaCO3.
General Chemistry Online! What is the buffer system in buffered aspirin?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/acidbase/faq/buffered-aspirin.shtml