
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
| Everyday chemistry |
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Chemistry of everyday life Chemistry of everyday life | Print | Comment |
| Previous Question | Next Question |
How does photographer's hypo work?
- Is AgCl solid soluble in Na2S2O3 aqueous?, if so why? If Ag solid is present will a developer coninue to reduce to Ag ions? if so why?
Elizabeth Brown browng@babe.net.au -
Converting Ag into Ag+ is oxidation, not reduction.
An emulsion of sodium thiosulfate (called hypo by photographers) is used to stop development of exposed film. Thiosulfate converts undeveloped silver bromide grains in the film into water-soluble silver thiosulfate complexes that can be removed when the film is washed.
S2O32-+ AgBr(s) Silver chloride behaves as silver bromide does.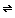 AgS2O3- + Br-
AgS2O3- + Br-
S2O32-+ AgS2O3-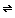 Ag(S2O3)3-
Ag(S2O3)3-
See these Web sites for more detail on the chemistry of photography:
-
Chemistry in Black and White Photography
(Shane Phillips, California State University)
Components of Photographic Materials
A laboratory exercise showing how photographic developers work. Includes a section on toning for iron, copper, and sepia.
http://wwwchem.csustan.edu/chem1002/photog.htm (12/27/98)(George L. Smyth) 
The composition and preparation of film paper, and developing chemicals is described.
http://www.freeyellow.com/members6/glsmyth/photomat.htm (3/19/99)
General Chemistry Online! How does photographer's hypo work?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/consumer/faq/photographers-hypo.shtml