
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
 Glossary
GlossaryGlossary: A
- ab initio.
- A calculation or prediction that is based purely on theory rather than on experimental data. Accurate ab initio predictions are an important test of a theory. (Lat., "from first principles")
- abrasive.
- A very hard, brittle, heat-resistant substance that is used to grind the edges or rough surfaces of an object. boron carbide, diamond, and corundum are abrasives.
- absolute error. absolute uncertainty. Compare with relative error
 .
. - The uncertainty in a measurement, expressed with appropriate units. For example, if three replicate weights for an object are 1.00 g, 1.05 g, and 0.95 g, the absolute error can be expressed as ± 0.05 g. Absolute error is also used to express inaccuracies; for example, if the "true value" is 1.11 g and the measured value is 1.00 g, the absolute error could be written as 1.00 g - 1.11 g = -0.11 g. Note that when absolute errors are associated with indeterminate errors
 , they are preceded with "±"; when they are associated with determinate errors
, they are preceded with "±"; when they are associated with determinate errors , they are preceded by their sign.
, they are preceded by their sign. - absolute temperature.
- Temperature measured on a scale that sets absolute zero
 as zero. In the SI
as zero. In the SI system, the kelvin
system, the kelvin scale is used to measure absolute temperature.
scale is used to measure absolute temperature. - absolute zero.
 (0 K)
(0 K) - The temperature at which the volume of an ideal gas
 becomes zero; a theoretical coldest temperature that can be approached but never reached. Absolute zero is zero on the Kelvin scale, -273.15°C on the Celsius
becomes zero; a theoretical coldest temperature that can be approached but never reached. Absolute zero is zero on the Kelvin scale, -273.15°C on the Celsius scale, and -459.67°F on the Fahrenheit scale.
scale, and -459.67°F on the Fahrenheit scale. - absorbance.
 (A, D, E) optical density; extinction; decadic absorbance.
(A, D, E) optical density; extinction; decadic absorbance. - A measure of the amount of light absorbed by a sample. The absorbance (A) equals minus the base-10 log of the transmittance
 .
. - absorption.
 absorb; absorbent. Compare with adsorption
absorb; absorbent. Compare with adsorption and sorption
and sorption .
. - 1. Penetration of molecules into the bulk of a solid or liquid, forming either a solution or compound. Absorption can be a chemical process (a strong solution of NaOH absorbs CO2 from the air) or a physical process (palladium absorbs hydrogen gas). 2. Capture and transformation of energy by a substance; for example, copper looks reddish because it absorbs blue light. An absorbent captures another material and distributes it throughout; an adsorbent captures another material and distributes it on its surface only.
- absorption spectroscopy.
 Compare with absorption spectrum
Compare with absorption spectrum .
. - A technique for determining the concentration and structure of a substance by measuring the amound of electromagnetic radiation
 the sample absorbs at various wavelengths
the sample absorbs at various wavelengths .
. - absorption spectrum. absorption spectra. Compare with absorption spectroscopy
 .
. - A plot that shows how much radiation a substance absorbs at different wavelengths
 . Absorption spectra are unique for each element and compound and they are often used as chemical "fingerprints" in analytical chemistry. The spectrum can represented by a plot of either absorbance
. Absorption spectra are unique for each element and compound and they are often used as chemical "fingerprints" in analytical chemistry. The spectrum can represented by a plot of either absorbance or transmittance
or transmittance versus wavelength, frequency
versus wavelength, frequency , or wavenumber
, or wavenumber .
. - absorptivity.
 (a) extinction coefficient; absorption cross section; decadic absorptivity. Compare with molar absorptivity
(a) extinction coefficient; absorption cross section; decadic absorptivity. Compare with molar absorptivity and absorbance
and absorbance .
. - The absorbance
 of a solution per unit of path length
of a solution per unit of path length and per unit concentration; a = A/(bc) where a, A, b, and c are the absorptivity, absorbance, path length, and concentration, respectively. Absorptivity varies with wavelength
and per unit concentration; a = A/(bc) where a, A, b, and c are the absorptivity, absorbance, path length, and concentration, respectively. Absorptivity varies with wavelength of the incident light.
of the incident light. - accelerator.
- 1. A substance that makes vulcanization
 of rubber occur more quickly or at a lower temperature. 2. A substance that makes crosslinking
of rubber occur more quickly or at a lower temperature. 2. A substance that makes crosslinking in a polymer
in a polymer occur more quickly or at a lower temperature, e. g., accelerators are added to Super Glue to make it set up quickly.
occur more quickly or at a lower temperature, e. g., accelerators are added to Super Glue to make it set up quickly.
- accuracy.
 Compare with precision
Compare with precision and trueness
and trueness .
. - Accuracy is the correctness of a single measurement. The accuracy of a measurement is assessed by comparing the measurement with the true or accepted value, based on evidence independent of the measurement. The closeness of an average to a true value is referred to as "trueness".
- acetate.

 (CH3COO-, C2H3O2-) acetate ion.
(CH3COO-, C2H3O2-) acetate ion. - 1. an ion formed by removing the acidic hydrogen of acetic acid
 , HC2H3O2. 2. a compound derived by replacing the acidic hydrogen in acetic acid. 3. A fiber made of cellulose
, HC2H3O2. 2. a compound derived by replacing the acidic hydrogen in acetic acid. 3. A fiber made of cellulose acetate.
acetate. - acetic acid

 (CH3COOH, HC2H3O2) ethanoic acid; vinegar acid; methanecarboxylic acid.
(CH3COOH, HC2H3O2) ethanoic acid; vinegar acid; methanecarboxylic acid. - A simple organic acid that gives vinegar its characteristic odor and flavor. Glacial acetic acid is pure acetic acid.
- acid.
 ([Lat. acidus, sour]) Compare with base
([Lat. acidus, sour]) Compare with base .
. - 1. a compound which releases hydrogen ions (H+) in solution (Arrhenius). 2. a compound containing detachable hydrogen ions (Bronsted-Lowry). 3. a compound that can accept a pair of electrons from a base (Lewis)..
- acid anhydride.
 Compare with acid
Compare with acid .
. - Nonmetallic oxides or organic compounds that react with water to form acids
 . For example, SO2, CO2, P2O5, and SO3 are the acid anhydrides of sulfurous, carbonic, phosphoric, and sulfuric acids, respectively. Acetic anhydride (CH3CO)2O) reacts with water to form acetic acid.
. For example, SO2, CO2, P2O5, and SO3 are the acid anhydrides of sulfurous, carbonic, phosphoric, and sulfuric acids, respectively. Acetic anhydride (CH3CO)2O) reacts with water to form acetic acid. - acid-base indicator.
- A weak acid that has acid and base forms with sharply different colors. Changes in pH
 around the acid's pKa
around the acid's pKa are "indicated" by color changes.
are "indicated" by color changes. - acid dissociation constant.
 (Ka) acid ionization constant. Compare with base hydrolysis constant
(Ka) acid ionization constant. Compare with base hydrolysis constant .
. - The equilibrium constant
 for the dissociation of an acid into a hydrogen ion and an anion. For example, the acid dissociation constant for acetic acid is the equilibrium constant for HC2H3O2(aq)
for the dissociation of an acid into a hydrogen ion and an anion. For example, the acid dissociation constant for acetic acid is the equilibrium constant for HC2H3O2(aq)  H+(aq) + C2H3O2-(aq), which is Ka = [H+][C2H3O2-]/[HC2H3O2].
H+(aq) + C2H3O2-(aq), which is Ka = [H+][C2H3O2-]/[HC2H3O2]. - acid error. Compare with alkaline error
 .
. - A systematic error
 that occurs when glass pH
that occurs when glass pH electrodes are used in strongly acidic solutions. Glass electrodes give pH readings that are consistently too high in these solutions.
electrodes are used in strongly acidic solutions. Glass electrodes give pH readings that are consistently too high in these solutions. - acid halide. acid chloride; acyl halide; acyl chloride.
- Compounds containing a carbonyl
 group bound to a halogen
group bound to a halogen atom.
atom. - acidic solution.
- A solution in which the hydrogen ion
 activity
activity is higher than that of the hydroxide ion
is higher than that of the hydroxide ion , when the solvent
, when the solvent is water.
is water.
- acidulant.
- A substance added to food or beverages to lower pH
 and to impart a tart, acid taste. Phosphoric acid is an acidulant added to cola drinks.
and to impart a tart, acid taste. Phosphoric acid is an acidulant added to cola drinks. - actinide.
- Elements 89-102 are called actinides. Electrons added during the Aufbau construction
 of actinide atoms go into the 5f subshell. Actinides are unstable and undergo radioactive decay
of actinide atoms go into the 5f subshell. Actinides are unstable and undergo radioactive decay . The most common actinides on Earth are uranium and thorium.
. The most common actinides on Earth are uranium and thorium. - activated charcoal.
 activated carbon; active carbon.
activated carbon; active carbon. - A porous form of carbon that acts as a powerful adsorbent
 , used to decolorize liquids, recover solvents, and remove toxins from water and air.
, used to decolorize liquids, recover solvents, and remove toxins from water and air. - activated complex. transition state.
- An intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy
 is the difference between the energies of the activated complex and the reactants.
is the difference between the energies of the activated complex and the reactants. - activation energy.
 (Ea)
(Ea) - The minimum energy required to convert reactants into products; the difference between the energies of the activated complex
 and the reactants.
and the reactants. - active site.
- A pocket or crevice on an enzyme
 molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy
molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction.
for reaction. - activity. (a)
- An effective concentration used in thermodynamic calculations in place of the actual concentration to allow equations developed for ideal solutions
 to be used to treat real solutions.
to be used to treat real solutions. - activity coefficient. (
 )
) - The ratio of activity
 to concentration; a =
to concentration; a =  c where a,
c where a,  , and c are the activity, activity coefficient, and concentrations, respectively. Activity coefficients are usually obtained from measurements of the emf of electrochemical cells
, and c are the activity, activity coefficient, and concentrations, respectively. Activity coefficients are usually obtained from measurements of the emf of electrochemical cells or the colligative properties
or the colligative properties of solutions.
of solutions.
- adiabat. adiabatic line. Compare with adiabatic
 .
. - A line on an indicator diagram
 that represents an adiabatic
that represents an adiabatic process.
process. - adiabatic.
 adiabatic process; isentropic process.
adiabatic process; isentropic process. - A process that neither absorbs nor releases energy into the surroundings. For example, a chemical reaction taking place in a closed thermos bottle can be considered adiabatic. Very fast processes can often be considered adiabatic with respect to heat exchange with the surroundings, because heat exchange is not instantaneous.
- adiabatic ionization energy. Compare with vertical ionization energy
 .
. - The lowest energy required to remove an electron from an atom, ion, or molecule in the gas phase. The adiabatic ionization energy is the difference between the ground state
 energy of the ion formed and the energy of the original atom, molecule, or ion.
energy of the ion formed and the energy of the original atom, molecule, or ion.
- addition compound.
 complex compound. Compare with hydrate
complex compound. Compare with hydrate .
. - An addition compound contains two or more simpler compounds that can be packed in a definite ratio into a crystal. A dot is used to separate the compounds in the formula. For example, ZnSO4·7 H2O is an addition compound of zinc sulfate and water. This represents a compound, and not a mixture, because there is a definite 1:7 ratio of zinc sulfate to water in the compound. Hydrates
 are a common type of addition compound.
are a common type of addition compound. - adhesion.
 (cohesion)
(cohesion) - Attraction between different substances on either side of a phase boundary
 .
. - adsorb. adsorbed; adsorbing.
- To collect molecules of a substance on a surface.
- adsorbent.
 Compare with absorbent
Compare with absorbent .
. - A substance that collects molecules of another substance on its surface. For example, gases that make water taste bad are strongly adsorbed on activated charcoal
 granules in water filters.
granules in water filters. - adsorption.
 adsorb; adsorbed. Compare with absorption
adsorb; adsorbed. Compare with absorption and sorption
and sorption .
. - Adsorption is collection of a substance on the surface of a solid or a liquid. For example, gases that make water taste bad are strongly adsorbed on charcoal granules in water filters.
- adsorption chromatography.
- A technique for separating or analyzing mixtures that contain at least one component that is preferentially adsorbed
 by the stationary phase
by the stationary phase as it moves over it.
as it moves over it. - adsorption indicator.

- A substance that indicates an excess of a reactant in a precipitation
 reaction. For example, dichlorofluorescein is added to an NaCl solution being titrated with silver nitrate. Before the endpoint
reaction. For example, dichlorofluorescein is added to an NaCl solution being titrated with silver nitrate. Before the endpoint , excess chloride ions make the surface of the AgCl precipitate
, excess chloride ions make the surface of the AgCl precipitate negative, and dichlorofluorescein anions remain in solution. After the endpoint, the excess silver ions make the surface of the AgCl precipitate positive, and the dichlorofluorescein anions are adsorbed
negative, and dichlorofluorescein anions remain in solution. After the endpoint, the excess silver ions make the surface of the AgCl precipitate positive, and the dichlorofluorescein anions are adsorbed onto their surface. Adsorption changes the color of the indicator from yellow-green to pink.
onto their surface. Adsorption changes the color of the indicator from yellow-green to pink. - aeration.
 aerate.
aerate. - Preparation of a saturated solution
 of air gases by either spraying the solution in air or by bubbling air through it.
of air gases by either spraying the solution in air or by bubbling air through it. - aerosol.
 Compare with colloid
Compare with colloid .
. - A colloid
 in which solid particles or liquid droplets are suspended in a gas. Smoke is an example of a solid aerosol; fog is an example of a liquid aerosol.
in which solid particles or liquid droplets are suspended in a gas. Smoke is an example of a solid aerosol; fog is an example of a liquid aerosol. - agar.

- A gel made from seaweed used to make salt bridges
 .
. - alanine.
 (A, CH3CH(NH2)COOH) Ala; alpha-aminopropionic acid.
(A, CH3CH(NH2)COOH) Ala; alpha-aminopropionic acid. - A naturally occurring aliphatic
 amino acid
amino acid which is required for protein synthesis but is not essential in the diet. Beta-alanine (NH2CH2CH2COOH) also occurs naturally.
which is required for protein synthesis but is not essential in the diet. Beta-alanine (NH2CH2CH2COOH) also occurs naturally. - alcohol.
 (ROH) Compare with phenol
(ROH) Compare with phenol and hydroxide
and hydroxide .
. - An alcohol is an organic compound with a carbon bound to a hydroxyl
 group. Examples are methanol, CH3OH; ethanol, CH3CH2OH; propanol, CH3CH2CH2OH. Compounds with -OH attached to an aromatic ring
group. Examples are methanol, CH3OH; ethanol, CH3CH2OH; propanol, CH3CH2CH2OH. Compounds with -OH attached to an aromatic ring are called phenols
are called phenols rather than alcohols.
rather than alcohols. - aldehyde.
 (RCHO)
(RCHO) - An aldehyde is an organic compound with a carbon bound to a -(C=O)-H group. Examples are formaldehyde (HCHO), acetaldehyde, CH3CHO, and benzaldehyde, C6H6CHO.
- aliphatic.
 Compare with aromatic
Compare with aromatic .
. - An organic compound that does not contain ring structures.
- aliquot.

- A sample of precisely determined amount taken from a material.
- alkali metal.
 (alkaline earth metal) alkali metal element.
(alkaline earth metal) alkali metal element. - The Group 1 elements, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) react with cold water for form strongly alkaline hydroxide solutions, and are referred to as "alkali metals". Hydrogen is not considered an alkali metal, despite its position on some periodic tables.
- alkaline.

- Having a pH
 greater than 7.
greater than 7. - alkaline earth.

- An oxide of an alkaline earth metal
 , which produces an alkaline
, which produces an alkaline solution in reaction with water.
solution in reaction with water. - alkaline earth metal.
 (alkali metal)
(alkali metal) - The Group 2 elements, beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) form alkaline oxides and hydroxides and are called "alkaline earth metals".
- alkaline error. Compare with acid error
 .
. - A systematic error
 that occurs when glass electrodes are used to read the pH
that occurs when glass electrodes are used to read the pH of an extremely alkaline solution
of an extremely alkaline solution ; the electrode responds to sodium ions as though they were hydrogen ions, giving a pH reading that is consistently too low.
; the electrode responds to sodium ions as though they were hydrogen ions, giving a pH reading that is consistently too low. - alkalinity.

- A measure of a material's ability to neutralize acids
 . Alkalinities are usually determined using titration
. Alkalinities are usually determined using titration .
. - alkaloid.

- A class of bitter-tasting, basic organic compounds with nitrogen-containing rings. Alkaloids often have powerful effects on living things. Examples are cocaine, nicotine, strychnine, caffeine, and morphine.
- alkane.
 paraffin. Compare with hydrocarbon
paraffin. Compare with hydrocarbon and alkene
and alkene .
. - A series of organic
 compounds with general formula CnH2n+2. Alkane names end with -ane. Examples are propane
compounds with general formula CnH2n+2. Alkane names end with -ane. Examples are propane (with n=3) and octane
(with n=3) and octane (with n=8).
(with n=8). - alkene.

- A compound that consists of only carbon and hydrogen, that contains at least one carbon-carbon double bond. Alkene names end with -ene. Examples are ethylene (CH2=CH2); 1-propene (CH2=CH2CH3), and 2-octane (CH3CH2=CH2(CH2)4CH3).
- alkoxide.
 (RO- M+) alkoxide ion.
(RO- M+) alkoxide ion. - An ionic compound
 formed by removal of hydrogen ions from the hydroxyl
formed by removal of hydrogen ions from the hydroxyl group in an alcohol
group in an alcohol using reactive metals, e. g. sodium. For example, potassium metal reacts with methanol (CH3OH) to produce potassium methoxide (KOCH3).
using reactive metals, e. g. sodium. For example, potassium metal reacts with methanol (CH3OH) to produce potassium methoxide (KOCH3). - alkyl.
 (-CnH2n+1) alkyl group.
(-CnH2n+1) alkyl group. - A molecular fragment derived from an alkane
 by dropping a hydrogen atom from the formula. Examples are methyl (CH3) and ethyl (CH2CH3).
by dropping a hydrogen atom from the formula. Examples are methyl (CH3) and ethyl (CH2CH3). - alkyl halide.

- An alkyl group
 attached to a halogen
attached to a halogen atom.
atom.
- alkyne.

- A compound that consists of only carbon and hydrogen, that contains at least one carbon-carbon triple bond. Alkyne names end with -yne. Examples are acetylene (CH
 CH); 1-propyne (CH2
CH); 1-propyne (CH2 CH2CH3), and 2-octyne (CH3CH2
CH2CH3), and 2-octyne (CH3CH2 CH2(CH2)4CH3).
CH2(CH2)4CH3). - allo-.

- A prefix that designates the more stable of a pair of geometric isomers
 . allo- is sometimes used less precisely to designate isomers or close relatives of a compound.
. allo- is sometimes used less precisely to designate isomers or close relatives of a compound. - allobar.

- A form of an element that has isotopic abundances
 that are different from the naturally occuring form. For example, "depleted" uranium has had most of the uranium-235 removed, and is an allobar of natural uranium.
that are different from the naturally occuring form. For example, "depleted" uranium has had most of the uranium-235 removed, and is an allobar of natural uranium. - allomer.
 allomerism.
allomerism. - Substances with different chemical composition but the same crystalline form.
- allosteric effect. allosteric interaction.
- A change in the behavior of one part of a molecule caused by a change in another part of the molecule.
- allotrope.
 allotropy; allotropic; allotropism. Compare with isotope
allotropy; allotropic; allotropism. Compare with isotope and polymorph
and polymorph .
. - Some elements occur in several distinct forms called allotropes. Allotropes have different chemical and physical properties. For example, graphite and diamond are allotropes of carbon.
- alloy.
 alloying; alloyed. Compare with amalgam
alloying; alloyed. Compare with amalgam .
. - A mixture containing mostly metals. For example, brass is an alloy of copper and zinc. Steel contains iron and other metals, but also carbon.
- allyl. allylic; allyl group; allyl radical.
- A molecular fragment derived by removing a methyl hydrogen from propene (-CH2-CH2=CH2). For example, "allyl chloride" is 3-chloropropene, Cl-CH2-CH2=CH2.
- alpha particle.
 (42He)
(42He) - A particle that is commonly ejected from radioactive
 nuclei, consisting of two protons and two neutrons. Alpha particles are helium nuclei. Alpha particles have a mass of 6.644 655 98 × 10-27 kg or 4.001 506 1747 atomic mass units
nuclei, consisting of two protons and two neutrons. Alpha particles are helium nuclei. Alpha particles have a mass of 6.644 655 98 × 10-27 kg or 4.001 506 1747 atomic mass units . [1998 CODATA values]
. [1998 CODATA values] - alpha ray.
 (
( -ray) alpha radiation.
-ray) alpha radiation. - A stream of alpha particles
 . Alpha rays rapidly dissipate their energy as they pass through materials, and are far less penetrating than beta particles
. Alpha rays rapidly dissipate their energy as they pass through materials, and are far less penetrating than beta particles and gamma rays
and gamma rays .
. - amalgam.
 Compare with alloy
Compare with alloy .
. - An alloy
 that contains mercury.
that contains mercury. - American Chemical Society ACS.
- A large and influential professional society for professionals and students in chemistry and related fields.
- amide.

- An amide is an organic compound that contains a carbonyl
 group bound to nitrogen:
group bound to nitrogen: 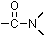 . The simplest amides are formamide (HCONH2) and acetamide (CH3CONH2).
. The simplest amides are formamide (HCONH2) and acetamide (CH3CONH2). - amine.
 Compare with ammine
Compare with ammine .
. - An amine is an organic compound that contains a nitrogen atom bound only to carbon and possibly hydrogen atoms. Examples are methylamine, CH3NH2; dimethylamine, CH3NHCH3; and trimethylamine, (CH3)3N.
- amino acid.

- Amino acids are molecules that contain at least one amine group (-NH2) and at least one carboxylic acid group (-COOH). When these groups are both attached to the same carbon, the acid is an
 -amino acid.
-amino acid.  -amino acids are the basic building blocks of proteins.
-amino acids are the basic building blocks of proteins. - ammine.
 Compare with amine
Compare with amine .
. - A metal ion complex containing ammonia
 as a ligand
as a ligand . The ammonia nitrogen is bound directly to a metal ion in ammines; amines differ in that the ammonia nitrogen is directly bound to a carbon atom.
. The ammonia nitrogen is bound directly to a metal ion in ammines; amines differ in that the ammonia nitrogen is directly bound to a carbon atom. - ammonia.

 (NH3) Compare with ammonium
(NH3) Compare with ammonium .
. - Pure NH3 is a colorless gas with a sharp, characteristic odor. It is easily liquified by pressure, and is very soluble in water. Ammonia acts as a weak base
 . Aqueous solutions of ammonia are (incorrectly) referred to as "ammonium hydroxide".
. Aqueous solutions of ammonia are (incorrectly) referred to as "ammonium hydroxide". - ammonium ion.

 (NH4+) ammonium.
(NH4+) ammonium. - NH4+ is a cation formed by neutralization of ammonia
 , which acts as a weak base
, which acts as a weak base .
. - amorphous.
 amorphous solid. Compare with crystal
amorphous solid. Compare with crystal .
. - A solid that does not have a repeating, regular three-dimensional arrangement of atoms, molecules, or ions.
- amperage.

- The amount of charge moved per second by an electric current
 , measured in amperes
, measured in amperes .
. - ampere.
 (A) amp.
(A) amp. - The SI
 unit of electric current
unit of electric current , equal to flow of 1 coulomb
, equal to flow of 1 coulomb of charge per second. An ampere is the amount of current necessary to produce a force of 0.2 micronewtons per meter between two arbitrarily long, arbitrarily thin wires, placed parallel in a vacuum and exactly 1 m apart. Named for 19th century physicist André Marie Ampère.
of charge per second. An ampere is the amount of current necessary to produce a force of 0.2 micronewtons per meter between two arbitrarily long, arbitrarily thin wires, placed parallel in a vacuum and exactly 1 m apart. Named for 19th century physicist André Marie Ampère. - amperometry. amperometric.
- Determining the concentration of a material in a sample by measuring electric current
 .
. - amphi-.
- A prefix used to name certain members of a series of geometric isomers
 or stereoisomers
or stereoisomers .
. - amphiprotic solvent. Compare with aprotic solvent
 .
. - Solvents that exhibit both acidic and basic properties; amphiprotic solvents undergo autoprotolysis
 . Examples are water, ammonia, and ethanol.
. Examples are water, ammonia, and ethanol. - amphoteric.
 ampholyte.
ampholyte. - A substance that can act as either an acid or a base in a reaction. For example, aluminum hydroxide can neutralize mineral acids ( Al(OH)3 + 3 HCl = AlCl3 + 3 H2O ) or strong bases ( Al(OH)3 + 3 NaOH = Na3AlO3 + 3 H2O).
- amplitude.

- The displacement of a wave from zero. The maximum amplitude for a wave is the height of a peak or the depth of a trough, relative to the zero displacement line.
- amylopectin. Compare with amylose
 .
. - A form of starch
 made of glucose molecules linked in a branching pattern.
made of glucose molecules linked in a branching pattern.
- amylose. Compare with amylopectin
 .
. - A form of starch
 made of long, unbranched chains of
made of long, unbranched chains of  -D-glucose molecules.
-D-glucose molecules.
- aprotic solvent. Compare with amphiprotic solvent
 .
. - A solvent that does not act as an acid or as a base; aprotic solvents don't undergo autoprotolysis
 . Examples are pentane, pet ether, and toluene.
. Examples are pentane, pet ether, and toluene. - analysis.
 chemical analysis.
chemical analysis. - Determination of the composition of a sample.
- analyte.
- An analyte is the sample constituent whose concentration is sought in a chemical analysis
 .
. - Angstrom.
 (Å) Ångstrom; Ångstrom units.
(Å) Ångstrom; Ångstrom units. - A non-SI
 unit of length used to express wavelengths of light, bond lengths, and molecular sizes. 1 Å = 10-10 m = 10-8 cm.
unit of length used to express wavelengths of light, bond lengths, and molecular sizes. 1 Å = 10-10 m = 10-8 cm. - angular momentum quantum number.
 (
( ) azimuthal quantum number; orbital angular momentum quantum number.
) azimuthal quantum number; orbital angular momentum quantum number. - A quantum number that labels the subshells
 of an atom. Sometimes called the orbital angular momentum quantum number, this quantum number dictates orbital shape.
of an atom. Sometimes called the orbital angular momentum quantum number, this quantum number dictates orbital shape.  can take on values from 0 to n-1 within a shell
can take on values from 0 to n-1 within a shell with principal quantum number
with principal quantum number n.
n. - anhydrous.
 anhydrous compound; anhydride. Compare with hydrate
anhydrous compound; anhydride. Compare with hydrate .
. - A compound with all water removed, especially water of hydration. For example, strongly heating copper(II) sulfate pentahydrate (CuSO4·5H2O) produces anhydrous copper(II) sulfate (CuSO4).
- anion.
 Compare with cation
Compare with cation .
. - An anion is a negatively charged ion. Nonmetals
 typically form anions.
typically form anions. - anode.
 Compare with cathode
Compare with cathode .
. - The electrode at which oxidation
 occurs in a cell. Anions
occurs in a cell. Anions migrate to the anode.
migrate to the anode. - anodize.

- To coat a metal with a protective film by electrolysis.
- anthocyanin. anthocyan.
- A family of pigments that give flowers, fruits, and leaves of some plants their red or blue coloring. Anthocyanins consist of sugar molecules bound to a benzopyrylium salt (called anthocyanidin). See Water to Wine for more about anthocyanins.
- antibonding orbital.
 antibonding; antibonding molecular orbital.
antibonding; antibonding molecular orbital. - A molecular orbital
 that can be described as the result of destructive interference
that can be described as the result of destructive interference of atomic orbitals
of atomic orbitals on bonded atoms. Antibonding orbitals have energies higher than the energies its constituent atomic orbitals would have if the atoms were separate.
on bonded atoms. Antibonding orbitals have energies higher than the energies its constituent atomic orbitals would have if the atoms were separate. - antichlor.

- A chemical compound that reacts with chlorine-based bleaches to stop the bleaching. Thiosulfate compounds are antichlors.
- antioxidant.

- Antioxidants are compounds that slow oxidation
 processes that degrade foods, fuels, rubber, plastic, and other materials. Antioxidants like butylated hydroxyanisole (BHA) are added to food to prevent fats from becoming rancid and to minimize decomposition of vitamins and essential fatty acids; they work by scavenging destructive free radicals
processes that degrade foods, fuels, rubber, plastic, and other materials. Antioxidants like butylated hydroxyanisole (BHA) are added to food to prevent fats from becoming rancid and to minimize decomposition of vitamins and essential fatty acids; they work by scavenging destructive free radicals from the food.
from the food. - antiozonant. antiozidant.
- Substances that reverse or prevent severe oxidation by ozone. Antiozonants are added to rubber to prevent them from becoming brittle as atmospheric ozone reacts with them over time. Aromatic
 amines
amines are often used as antiozonants.
are often used as antiozonants. - antipyretic.
- A substance that can lessen or prevent fever.
- Antoine equation Antoine's equation.
- A simple 3-parameter fit to experimental vapor pressures measured over a restricted temperature range:
where A, B, and C are "Antoine coefficients" that vary from substance to substance. Sublimations and vaporizations of the same substance have separate sets of Antoine coefficients, as do components in mixtures. The Antoine equation is accurate to a few percent for most volatile substances (with vapor pressures over 10 Torr).log P = A - B
T + C - aqua regia.

- A mixture of nitric and hydrochloric acids, usually 1:3 or 1:4 parts HNO3 to HCl, used to dissolve gold.
- aqueous.
 (aq) aqueous solution.
(aq) aqueous solution. - A substance dissolved in water.
- arene.

- A hydrocarbon
 that contains at least one aromatic ring
that contains at least one aromatic ring .
. - arginine.
 (R, C6H14N4O2) Arg.
(R, C6H14N4O2) Arg.  An essential amino acid
An essential amino acid and building block of proteins. Arginine acts as a base under physiological conditions; the double-bonded nitrogen on the end of the side chain readily captures a hydrogen ion, becoming positively charged. This charged side group makes arginine hydrophilic
and building block of proteins. Arginine acts as a base under physiological conditions; the double-bonded nitrogen on the end of the side chain readily captures a hydrogen ion, becoming positively charged. This charged side group makes arginine hydrophilic .
.- aromatic ring.
 (Ar)
(Ar) - An exceptionally stable planar ring of atoms with resonance structures that consist of alternating double and single bonds, e. g. benzene:
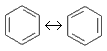
- aromatic compound.

- A compound containing an aromatic ring
 . Aromatic compounds have strong, characteristic odors.
. Aromatic compounds have strong, characteristic odors. - Arrhenius equation.
- In 1889, Svante Arrhenius explained the variation of rate constants
 with temperature for several elementary reactions
with temperature for several elementary reactions using the relationship
using the relationship k = A exp(-Ea/RT)
where the rate constant k is the total frequency of collisions between reaction molecules A times the fraction of collisions exp(-Ea/RT) that have an energy that exceeds a threshold activation energy
between reaction molecules A times the fraction of collisions exp(-Ea/RT) that have an energy that exceeds a threshold activation energy Ea at a temperature of T (in kelvins). R is the universal gas constant
Ea at a temperature of T (in kelvins). R is the universal gas constant .
. - aryl.
 (Ar) aryl group.
(Ar) aryl group. - A molecular fragment or group attached to a molecule by an atom that is on an aromatic ring
 .
. - asparagine.
 Asn.
Asn.  A natural amino acid
A natural amino acid that is the amide
that is the amide of aspartic acid
of aspartic acid .
.- aspartic acid.
 (D,HOOCCH2CH(NH2)COOH) Asp.
(D,HOOCCH2CH(NH2)COOH) Asp. - A nonessential amino acid
 that is abundant in molasses. The carboxylic acid
that is abundant in molasses. The carboxylic acid group on the side chain is ionized under physiological conditions, making aspartic acid residues in proteins hydrophilic
group on the side chain is ionized under physiological conditions, making aspartic acid residues in proteins hydrophilic .
. - atmosphere. (atm)
- A unit of pressure, equal to a barometer reading of 760 mm Hg. 1 atmosphere is 101325 pascals
 and 1.01325 bar
and 1.01325 bar .
. - atomic mass unit.
 (amu,u) amu; dalton.
(amu,u) amu; dalton. - A unit of mass equal to 1/12 the mass of a carbon-12 nucleus, which is 1.660 538 73 × 10-27 kg ± 0.000 000 13 × 10-27 kg [1998 CODATA values]. Abbreviated as amu or u. Sometimes called the dalton, after John Dalton, architect of the first modern atomic theory.
- atomic nucleus.
 nucleus; nuclei; atomic nuclei.
nucleus; nuclei; atomic nuclei. - A tiny, incredibly dense positively charged mass at the heart of the atom. The nucleus is composed of protons
 and neutrons
and neutrons (and other particles). It contains almost all of the mass of the atom but occupies only a tiny fraction of the atom's volume.
(and other particles). It contains almost all of the mass of the atom but occupies only a tiny fraction of the atom's volume. - atomic number.
 (Z)
(Z) - The number of protons
 in an atomic nucleus
in an atomic nucleus . The atomic number and the element symbol
. The atomic number and the element symbol are two alternate ways to label an element. In nuclide symbols
are two alternate ways to label an element. In nuclide symbols , the atomic number is a leading subscript; for example, in 126C, the "6" is the atomic number.
, the atomic number is a leading subscript; for example, in 126C, the "6" is the atomic number. - atomic orbital.

- A wavefunction
 that describes the behavior of an electron in an atom.
that describes the behavior of an electron in an atom. - atomic radius.
 metallic radius; covalent radius; atomic radii. Compare with ionic radius
metallic radius; covalent radius; atomic radii. Compare with ionic radius .
. - One half the distance between nuclei of atoms of the same element, when the atoms are bound by a single covalent bond or are in a metallic crystal. The radius of atoms obtained from covalent bond lengths is called the covalent radius; the radius from interatomic distances in metallic crystals is called the metallic radius.
- atomic theory.
- An explanation of chemical properties and processes that assumes that tiny particles called atoms are the ultimate building blocks of matter.
- atomic unit. Compare with Bohr radius
 and hartree
and hartree .
. - A system of non-SI
 units used in quantum chemistry to simplify calculations and mathematical expressions. The definitions of atomic units include physical constants (like the speed of light
units used in quantum chemistry to simplify calculations and mathematical expressions. The definitions of atomic units include physical constants (like the speed of light , the rest mass of the electron, and other quantities that never change), so that all constants drop out of expressions when atomic units are used.
, the rest mass of the electron, and other quantities that never change), so that all constants drop out of expressions when atomic units are used.
- atomic weight.
 atomic mass.
atomic mass. - The average mass of an atom of an element, usually expressed in atomic mass units
 . The terms mass
. The terms mass and weight
and weight are used interchangeably in this case. The atomic weight given on the periodic table is a weighted average of isotopic masses
are used interchangeably in this case. The atomic weight given on the periodic table is a weighted average of isotopic masses found in a typical terrestrial sample of the element.
found in a typical terrestrial sample of the element. - atom.
 Compare with molecule
Compare with molecule and ion
and ion .
. - An atom is the smallest particle of an element that retains the chemical properties of the element. Atoms are electrically neutral, with a positively charged nucleus that binds one or more electrons in motion around it.
- atto-. (a)
- Prefix used in the SI
 system meaning "multiply by 10-18". For example, 3 am means 3× 10-18 meters.
system meaning "multiply by 10-18". For example, 3 am means 3× 10-18 meters. - aufbau principle. aufbau construction; building-up principle.
- An approximate procedure for writing the ground state
 electronic configuration
electronic configuration of atoms. The configuration of an atom is obtained by inserting one electron into the configuration of the atom immediately to its left on the periodic table. The electron is inserted into the subshell indicated by the element's period
of atoms. The configuration of an atom is obtained by inserting one electron into the configuration of the atom immediately to its left on the periodic table. The electron is inserted into the subshell indicated by the element's period and block
and block .
. - auto-ignition temperature. Compare with flash point
 .
. - Minimum temperature at which the vapor/air mixture over a liquid spontaneously catches fire.
- autoxidation. autooxidation; autoxidize; autoxidizing.
- Oxidation
 caused by exposure to air. Rust is an example of autoxidation. Autoxidation makes ether taken from half-filled bottles very dangerous, because air oxidizes ether to highly explosive organic peroxides.
caused by exposure to air. Rust is an example of autoxidation. Autoxidation makes ether taken from half-filled bottles very dangerous, because air oxidizes ether to highly explosive organic peroxides. - autoprotolysis.
 autoionization; autoionization constant; autoprotolysis constant.
autoionization; autoionization constant; autoprotolysis constant. - Transfer of a hydrogen ion between molecules of the same substance, e. g. the autoprotolysis of methanol (2 CH3OH = CH3OH2+ + CH3O-). Autoprotolysis of water into hydronium
 ions and hydroxide ions
ions and hydroxide ions results in equilibrium concentrations that satisfy Kw = [H3O+][OH-], where the autoprotolysis constant Kw is equal to 1.01 × 10-14 at 25°C.
results in equilibrium concentrations that satisfy Kw = [H3O+][OH-], where the autoprotolysis constant Kw is equal to 1.01 × 10-14 at 25°C. - auxochrome. Compare with chromophore
 .
. - A group or substructure in a molecule that influences the intensity of absorption
 of the molecule.
of the molecule. - average bond enthalpy.
 Compare with bond enthalpy
Compare with bond enthalpy .
. - Average enthalpy change per mole when the same type of bond is broken in the gas phase for many similar substances
- Avogadro.
 Amadeo Avogadro.
Amadeo Avogadro. - Italian chemist and physicist Amadeo Avogadro (1776-1856) proposed a correct molecular explanation for Gay-Lussac's law of combining volumes
 . His work provided a simple way to determine atomic weights
. His work provided a simple way to determine atomic weights and molecular weights
and molecular weights of gases.
of gases. - Avogadro number.
 (NA, L) Avogadro's number; Avogadro constant.
(NA, L) Avogadro's number; Avogadro constant. - The number of particles in one mole, equal to 6.02214199 × 1023 mol-1 (± 0.00000047 mol-1) [1998 CODATA values]
- Avogadro's law.

- Equal volumes of an ideal gas
 contain equal numbers of molecules, if both volumes are at the same temperature and pressure. For example, 1 L of ideal gas contains twice as many molecules as 0.5 L of ideal gas at the same temperature and pressure.
contain equal numbers of molecules, if both volumes are at the same temperature and pressure. For example, 1 L of ideal gas contains twice as many molecules as 0.5 L of ideal gas at the same temperature and pressure. - axial.

- 1. An atom, bond, or lone pair
 that is perpendicular to equatorial
that is perpendicular to equatorial atoms, bonds, and lone pairs in a trigonal bipyramidal
atoms, bonds, and lone pairs in a trigonal bipyramidal molecular geometry
molecular geometry .
. - azeotrope.
 azeotropic mixture; azeotropy.
azeotropic mixture; azeotropy. - A solution that does not change composition when distilled. For example, if a 95% (w/w) ethanol solution in water is boilled, the vapor produced also is 95% ethanol- and it is not possible to obtain higher percentages of ethanol by distillation.
- azo.
 azo compound; azo group; azo dye.
azo compound; azo group; azo dye. - The azo group has the general structure Ar-N=N-Ar', where Ar and Ar' indicate substituted aromatic rings
 . Compounds containing the azo compounds are often intensely colored and are economically important as dyes. Methyl orange is an example of an azo dye.
. Compounds containing the azo compounds are often intensely colored and are economically important as dyes. Methyl orange is an example of an azo dye.

General Chemistry Online! Glossary: A
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/glossary/a.shtml