
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
| Energy & change |
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Energy and chemical change Energy and chemical change | Print | Comment |
| Next Question |
How do I calculate calorimeter heat capacities from experimental data?
- How do I calculate the heat capacity of a calorimeter if 93.3 g iron at 65.58°C is placed in 75.0g of water, raising the temp from 16.95°C to 19.68°C for the system?
Annie -
Annie, here's the basic strategy for finding the heat capacity of the calorimeter (sometimes called the calorimeter constant):
- Identify all heats transfers by deciding which parts of the system absorb or release significant amounds of heat.
In your problem, you have three heats:
- Heat is released by the 93.3 g of iron. Call it q(iron).
- Heat is absorbed by the 75.0 g of water. Call it q(water).
- Heat is absorbed by the calorimeter. Call it q(calorimeter).
- Set up an energy conservation equation. The sum of all the heat flows listed above is zero.
q(iron) + q(water) + q(calorimeter) = 0
This is an approximation. When an object warms, it expands, and it has to push against the atmosphere to do that. That work against the atmosphere involves a bit of energy that should be included in the equation. We ignore it because it's small in this case and it's much simpler to leave it out. the things that are mentioned in the problem.
- introduce
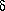 T's. Replace experimental q's with temperature changes,
using q = mc
T's. Replace experimental q's with temperature changes,
using q = mc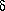 T or q = C
T or q = C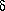 T, where c is the specific heat and C is the heat capacity. For your problem, the energy conservation equation becomes
T, where c is the specific heat and C is the heat capacity. For your problem, the energy conservation equation becomes
m(iron) c(iron)
You can see that you'll need to look up the specific heat of iron and of water to finish the problem.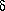 T(iron) + m(water) c(water)
T(iron) + m(water) c(water) 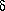 T(water) + C(calorimeter)
T(water) + C(calorimeter) 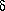 T(calorimeter) = 0
T(calorimeter) = 0
- Solve the equation for the desired quantity. Do a bit of algebra and get C(calorimeter) all by itself on one side of the equation, and you're done.
- Identify all heats transfers by deciding which parts of the system absorb or release significant amounds of heat.
In your problem, you have three heats:
General Chemistry Online! How do I calculate calorimeter heat capacities from experimental data?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/thermo/faq/calorimeter-constant.shtml