
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Companion Notes
Just Ask Antoine!
Resources
Slide Index
Toolbox
Tutorial Index
FAQ
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
| Acids & bases |
Reaction rates
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Acids and bases Acids and bases | Print | Comment | Contact |
| Previous Question | Next Question |
How can strong and weak acids be distinguished using indicators?
- How can I tell if an unknown acid is strong or weak? I have only methyl orange, phenolphthalein, and bromothymol blue indicators.
Rei Rei 01/09/99 -
The titration curve
 for a strong acid
for a strong acid has a very large
jump in pH at the equivalence point
has a very large
jump in pH at the equivalence point , while the jump for a weak acid
, while the jump for a weak acid is much smaller.
This fact can be used to distinguish strong acids from weak provided the concentration of the acid is not much less than 0.1 M.
is much smaller.
This fact can be used to distinguish strong acids from weak provided the concentration of the acid is not much less than 0.1 M.
The rather gaudy diagram above shows the titration curve for a strong acid (40 mL of 0.1 M HCl, titrated with 0.1 M NaOH) in blue, and the titration curve for a weak acid (40 mL of 0.1 M HC2H3O2, Ka = 1.8×10-5) in black. The colored bands show the pH ranges at which indicator color changes occur.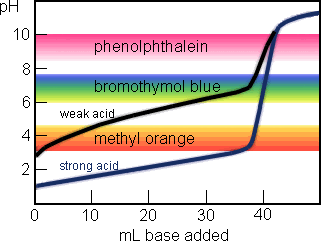
For the strong acid, the equivalence point occurs at pH 7.0. Bromothymol blue is the best of the three indicators for this titration. But the color changes for methyl orange , bromothymol blue, and phenolphthalein will all occur at nearly the same volume of added base.
For the weak acid, the methyl orange color change will occur long before the equivalence point, the bromothymol blue change is slightly premature, and phenolphthalein color change occurs close to the correct place (around pH 8.9). The weaker the acid is, the larger the discrepancy between the three indicator endpoints will be.
This method of distinguishing strong acids from weak ones won't work if the concentration of the acid and/or the titrant is much less than 0.1 M. Diluting the acid makes the "break" in the titration curve less steep and the indicator endpoints will differ even for strong acids in that case.
newsletter describing updates,
new features, and changes
on this site.
General Chemistry Online! How can strong and weak acids be distinguished using indicators?
Copyright © 1997-2001 by Fred Senese
Comments & questions to senese@antoine.frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101-hidden/acidbase/faq/indicating-strong-or-weak.shtml