
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Companion Notes
Just Ask Antoine!
Resources
Slide Index
Toolbox
Tutorial Index
FAQ
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
| Everyday chemistry |
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Chemistry of everyday life Chemistry of everyday life | Print | Comment | Contact |
| Previous Question | Next Question |
What is soap made of?
-
-
Soaps are made of molecules that are both fat and water soluble. The molecule has a long hydrocarbon tail
that allows it to dissolve grease, and a polar head that is water soluble.
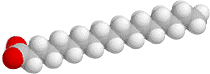 A soap molecule. The grey, red, and white balls represent carbon, oxygen, and hydrogen atoms, respectively. The sodium ion near the negatively charged oxygen atoms is not shown.
A soap molecule. The grey, red, and white balls represent carbon, oxygen, and hydrogen atoms, respectively. The sodium ion near the negatively charged oxygen atoms is not shown.
The head is the sodium or potassium salt of an organic acid.How do you make a molecule like this? Animal fat contains them, chemically bound to a small three-armed molecule called glycerol. The links between each of the three acid molecules and the glycerol are easily broken in a hot, alkaline solution:
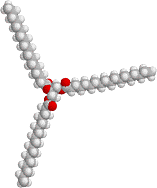
+ 3 NaOH 
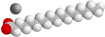
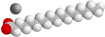
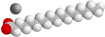
+ 
A glyceride (three fatty acids bound to a glycerol) three soap molecules glycerol 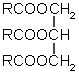
+ 3 NaOH 
3 RCOO- Na+ + 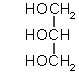
This reaction was used to make soap by treating animal fat with wood ash (which contain a high concentration of potassium hydroxide and potassium carbonate) long before the chemistry of the process was understood.
newsletter describing updates,
new features, and changes
on this site.
General Chemistry Online! What is soap made of?
Copyright © 1997-2001 by Fred Senese
Comments & questions to senese@antoine.frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101-hidden/consumer/faq/making-soap.shtml