
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Companion Notes
Just Ask Antoine!
Resources
Slide Index
Toolbox
Tutorial Index
FAQ
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
| Redox reactions |
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Redox reactions Redox reactions | Print | Comment | Contact |
| Previous Question | Next Question |
Why does a voltage develop at an electrode surface?
-
-
Think of the metal plates not as a lot of metal atoms packed together, but as a lot of metal ions floating in a sea of electrons. The boundary between the metal and a solution containing metal cations is pictured below.
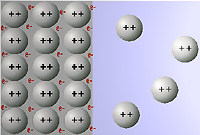 Notice that each of the metal ions has a different 'atmosphere' of charges around it. The cations in the metal are surrounded by electrons and other cations; the cations in the solution are surrounded mostly by water molecules (with other ions farther off). Atoms right on the boundary line will 'feel' different charges on the solution side and on the metal side. This is where the voltage comes from.
Notice that each of the metal ions has a different 'atmosphere' of charges around it. The cations in the metal are surrounded by electrons and other cations; the cations in the solution are surrounded mostly by water molecules (with other ions farther off). Atoms right on the boundary line will 'feel' different charges on the solution side and on the metal side. This is where the voltage comes from.
newsletter describing updates,
new features, and changes
on this site.
General Chemistry Online! Why does a voltage develop at an electrode surface?
Copyright © 1997-2001 by Fred Senese
Comments & questions to senese@antoine.frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101-hidden/redox/faq/why-electrode-voltages.shtml