
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Companion Notes
Just Ask Antoine!
Resources
Slide Index
Toolbox
Tutorial Index
FAQ
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
| Solutions |
Redox reactions
Reaction rates
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Solutions Solutions | Print | Comment | Contact |
| Previous Question | Next Question |
How do nonpolar molecules like CO2 and O2 dissolve in water?
-
-
How does O2 dissolve in water?
The oxygen has to cross the air/water interface- often a slow process. Just how slow depends on whether the water is still or running, how much surface is in contact with the air, what's dissolved in the water, and whether any films (like soap, or broken bacteria or algae cell walls) are floating on the surface. Once the oxygen crosses the surface, it is caged by water molecules.
How does CO2 dissolve in water? Here is a sketchy outline of the process. As with the O2, the CO2 must cross the surface of the liquid:
CO2(g)
It's a little easier for the CO2 to do so than for oxygen, because the oxygen ends of the molecule have a partial negative charge are better able to hydrogen-bond to the water as a result. The CO2 rather slowly acquires a shell of water molecules. A fraction of these hydrated carbon dioxide molecules react with the water to produce carbonic acid (H2CO3):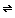 CO2(aq)
CO2(aq)
CO2(aq) + H2O
The equilibrium constant for this reaction is about 1.6×10-3 around room temperature, which means that most of the dissolved carbon dioxide is present as hydrated CO2. Only about 16% reacts with water to form carbonic acid. The reaction is rather slow. It involves bending a stable, linear CO2 molecule (with a water parked oxygen-down over the carbon) into a Y-shaped O=C(OH)2 molecule.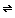 H2CO3(aq)
H2CO3(aq)
The carbonic acid is a weak acid, and it can dissociate to form bicarbonate ion (HCO3-) and (in basic solution) carbonate ion: (CO32-):
H2CO3(aq)
Notice that hydrogen ions are produced. That means that any water with dissolved CO2 will be slightly acidic. In fact, distilled water in equilibrium with air has a pH of around 5.7 and not 7.0, due to these reactions (air contains between 300 and 400 ppm of CO2). Check it out for yourself. Measure the pH of freshly distilled water. Let the same water sit for a day or two, and measure the pH again. You can show how slowly the process occurs by timing your measurements.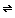 H+(aq) + HCO3-(aq)
H+(aq) + HCO3-(aq)
HCO3-(aq)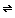 H+(aq) + CO32-(aq)
H+(aq) + CO32-(aq)
Because the carbonic acid forms slowly, adding a base to carbonated water causes the pH to jump up (as the small amount of carbonic acid is quickly neutralized) and then, slowly down again (as new carbonic acid forms from hydrated carbon dioxide). For a vivid demonstration of this point, see D. M. Kern's article "The Hydration of Carbon Dioxide", J. Chem. Ed., 37, 14 (1960).
Stephen Lower discusses these equilibria in Carbonate equilibria in natural waters (you'll need the Adobe Acrobat reader to view the document).
newsletter describing updates,
new features, and changes
on this site.
General Chemistry Online! How do nonpolar molecules like CO_2_ and O_2_ dissolve in water?
Copyright © 1997-2001 by Fred Senese
Comments & questions to senese@antoine.frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101-hidden/solutions/faq/dissolving-gases.shtml