
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Companion Notes
Just Ask Antoine!
Resources
Slide Index
Toolbox
Tutorial Index
FAQ
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
| Electrons in atoms |
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Electrons in atoms Electrons in atoms | Print | Comment | Contact |
| Previous Question | Next Question |
How can you rank transitions in order of increasing wavelength?
- Given a list of electronic transitions, how can you tell which one corresponds to the longest and shortest emission wavelengths?
Jamelia -
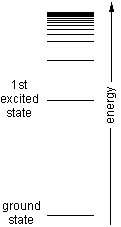 The energy ladder for a hydrogenlike atom is shown at left. There are three cases to consider:
The energy ladder for a hydrogenlike atom is shown at left. There are three cases to consider:
- If you're comparing two transitions with the same difference in quantum number, notice that the rungs on the bottom of the energy ladder are further apart than the rungs on top. That means that emission wavelengths are smaller for transitions low on the ladder.
- If you're comparing two transitions that involve the same quantum number, the one with the larger difference between quantum numbers will have the higher transition energy and the smaller wavelength.
- If you want to compare a pair of transitions that don't fit under (1) or (2), use the fact that the transition energies are proportional to (1/n22) - (1/n12), where n1 and n2 are the quantum numbers labelling the lower and upper states in the transition.
newsletter describing updates,
new features, and changes
on this site.
General Chemistry Online! How can you rank transitions in order of increasing wavelength?
Copyright © 1997-2001 by Fred Senese
Comments & questions to senese@antoine.frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101-hidden/electrons/faq/wavelength-transitions.shtml