
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
 Glossary
GlossaryGlossary: Introduction to organic chemistry
- acid anhydride.
 Compare with acid
Compare with acid .
. - Nonmetallic oxides or organic compounds that react with water to form acids
 . For example, SO2, CO2, P2O5, and SO3 are the acid anhydrides of sulfurous, carbonic, phosphoric, and sulfuric acids, respectively. Acetic anhydride (CH3CO)2O) reacts with water to form acetic acid.
. For example, SO2, CO2, P2O5, and SO3 are the acid anhydrides of sulfurous, carbonic, phosphoric, and sulfuric acids, respectively. Acetic anhydride (CH3CO)2O) reacts with water to form acetic acid. - acid halide. acid chloride; acyl halide; acyl chloride.
- Compounds containing a carbonyl
 group bound to a halogen
group bound to a halogen atom.
atom. - -al.
- A suffix added to the systematic names of organic compounds that contain an aldehyde
 group -(C=O)-H. For example, the systematic name of acetaldehyde, CH3CHO, is ethanal.
group -(C=O)-H. For example, the systematic name of acetaldehyde, CH3CHO, is ethanal. - alcohol.
 (ROH) Compare with phenol
(ROH) Compare with phenol and hydroxide
and hydroxide .
. - An alcohol is an organic compound with a carbon bound to a hydroxyl
 group. Examples are methanol, CH3OH; ethanol, CH3CH2OH; propanol, CH3CH2CH2OH. Compounds with -OH attached to an aromatic ring
group. Examples are methanol, CH3OH; ethanol, CH3CH2OH; propanol, CH3CH2CH2OH. Compounds with -OH attached to an aromatic ring are called phenols
are called phenols rather than alcohols.
rather than alcohols. - aldehyde.
 (RCHO)
(RCHO) - An aldehyde is an organic compound with a carbon bound to a -(C=O)-H group. Examples are formaldehyde (HCHO), acetaldehyde, CH3CHO, and benzaldehyde, C6H6CHO.
- aliphatic.
 Compare with aromatic
Compare with aromatic .
. - An organic compound that does not contain ring structures.
- alkane.
 paraffin. Compare with hydrocarbon
paraffin. Compare with hydrocarbon and alkene
and alkene .
. - A series of organic
 compounds with general formula CnH2n+2. Alkane names end with -ane. Examples are propane
compounds with general formula CnH2n+2. Alkane names end with -ane. Examples are propane (with n=3) and octane
(with n=3) and octane (with n=8).
(with n=8). - alkene.

- A compound that consists of only carbon and hydrogen, that contains at least one carbon-carbon double bond. Alkene names end with -ene. Examples are ethylene (CH2=CH2); 1-propene (CH2=CH2CH3), and 2-octane (CH3CH2=CH2(CH2)4CH3).
- alkoxide.
 (RO- M+) alkoxide ion.
(RO- M+) alkoxide ion. - An ionic compound
 formed by removal of hydrogen ions from the hydroxyl
formed by removal of hydrogen ions from the hydroxyl group in an alcohol
group in an alcohol using reactive metals, e. g. sodium. For example, potassium metal reacts with methanol (CH3OH) to produce potassium methoxide (KOCH3).
using reactive metals, e. g. sodium. For example, potassium metal reacts with methanol (CH3OH) to produce potassium methoxide (KOCH3). - alkyl.
 (-CnH2n+1) alkyl group.
(-CnH2n+1) alkyl group. - A molecular fragment derived from an alkane
 by dropping a hydrogen atom from the formula. Examples are methyl (CH3) and ethyl (CH2CH3).
by dropping a hydrogen atom from the formula. Examples are methyl (CH3) and ethyl (CH2CH3). - alkyne.

- A compound that consists of only carbon and hydrogen, that contains at least one carbon-carbon triple bond. Alkyne names end with -yne. Examples are acetylene (CH
 CH); 1-propyne (CH2
CH); 1-propyne (CH2 CH2CH3), and 2-octyne (CH3CH2
CH2CH3), and 2-octyne (CH3CH2 CH2(CH2)4CH3).
CH2(CH2)4CH3). - allo-.

- A prefix that designates the more stable of a pair of geometric isomers
 . allo- is sometimes used less precisely to designate isomers or close relatives of a compound.
. allo- is sometimes used less precisely to designate isomers or close relatives of a compound. - allyl. allylic; allyl group; allyl radical.
- A molecular fragment derived by removing a methyl hydrogen from propene (-CH2-CH2=CH2). For example, "allyl chloride" is 3-chloropropene, Cl-CH2-CH2=CH2.
- amide.

- An amide is an organic compound that contains a carbonyl
 group bound to nitrogen:
group bound to nitrogen: 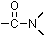 . The simplest amides are formamide (HCONH2) and acetamide (CH3CONH2).
. The simplest amides are formamide (HCONH2) and acetamide (CH3CONH2). - amine.
 Compare with ammine
Compare with ammine .
. - An amine is an organic compound that contains a nitrogen atom bound only to carbon and possibly hydrogen atoms. Examples are methylamine, CH3NH2; dimethylamine, CH3NHCH3; and trimethylamine, (CH3)3N.
- amino acid.

- Amino acids are molecules that contain at least one amine group (-NH2) and at least one carboxylic acid group (-COOH). When these groups are both attached to the same carbon, the acid is an
 -amino acid.
-amino acid.  -amino acids are the basic building blocks of proteins.
-amino acids are the basic building blocks of proteins. - ammine.
 Compare with amine
Compare with amine .
. - A metal ion complex containing ammonia
 as a ligand
as a ligand . The ammonia nitrogen is bound directly to a metal ion in ammines; amines differ in that the ammonia nitrogen is directly bound to a carbon atom.
. The ammonia nitrogen is bound directly to a metal ion in ammines; amines differ in that the ammonia nitrogen is directly bound to a carbon atom. - arene.

- A hydrocarbon
 that contains at least one aromatic ring
that contains at least one aromatic ring .
. - aromatic ring.
 (Ar)
(Ar) - An exceptionally stable planar ring of atoms with resonance structures that consist of alternating double and single bonds, e. g. benzene:
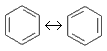
- aromatic compound.

- A compound containing an aromatic ring
 . Aromatic compounds have strong, characteristic odors.
. Aromatic compounds have strong, characteristic odors. - aryl.
 (Ar) aryl group.
(Ar) aryl group. - A molecular fragment or group attached to a molecule by an atom that is on an aromatic ring
 .
. - azo.
 azo compound; azo group; azo dye.
azo compound; azo group; azo dye. - The azo group has the general structure Ar-N=N-Ar', where Ar and Ar' indicate substituted aromatic rings
 . Compounds containing the azo compounds are often intensely colored and are economically important as dyes. Methyl orange is an example of an azo dye.
. Compounds containing the azo compounds are often intensely colored and are economically important as dyes. Methyl orange is an example of an azo dye. - carbonyl.
 carbonyl group.
carbonyl group. - A divalent
 group consisting of a carbon atom with a double-bond to oxygen. For example, acetone (CH3-(C=O)-CH3) is a carbonyl group linking two methyl
group consisting of a carbon atom with a double-bond to oxygen. For example, acetone (CH3-(C=O)-CH3) is a carbonyl group linking two methyl groups. Also refers to a compound of a metal with carbon monoxide
groups. Also refers to a compound of a metal with carbon monoxide , such as iron carbonyl, Fe(CO)5.
, such as iron carbonyl, Fe(CO)5. - carboxylic acid.
 carboxyl; carboxyl group.
carboxyl; carboxyl group. - A carboxylic acid is an organic molecule with a -(C=O)-OH group. The group is also written as -COOH and is called a carboxyl group. The hydrogen on the -COOH group ionizes in water; carboxylic acids are weak acids. The simplest carboxylic acids are formic acid (H-COOH) and acetic acid
 (CH3-COOH).
(CH3-COOH). - carotene.

- Carotene is an unsaturated
 hydrocarbon
hydrocarbon pigment found in many plants. Carotene is the basic building block of vitamin A.
pigment found in many plants. Carotene is the basic building block of vitamin A. - chelate.

- A stable complex of a metal with one or more polydentate
 ligands
ligands . For example, calcium complexes with EDTA
. For example, calcium complexes with EDTA to form a chelate.
to form a chelate. - chiral.
 chirality.
chirality. - Having nonsuperimposable mirror images. For example, a shoe or a glove is chiral.
- chiral center.
 asymmetric center.
asymmetric center. - An atom in a molecule that causes chirality
 , usually an atom that is bound to four different groups. A molecule can have chirality without having a chiral center, and a molecule may also have more than one chiral centers.
, usually an atom that is bound to four different groups. A molecule can have chirality without having a chiral center, and a molecule may also have more than one chiral centers. - conformers.
 conformation.
conformation. - Molecular arrangements that differ only by rotations around single bonds. For example, the "boat" and "chair" forms of cyclohexane are conformers.
- coordination number.

- The number of bonds formed by the central atom in a metal-ligand
 complex.
complex. - crystal field splitting energy. (
 )
) - Ligands
 complexed to a metal ion will raise the energy of some of its d orbitals and lower the energy of others. The difference in energy is called the crystal field splitting energy.
complexed to a metal ion will raise the energy of some of its d orbitals and lower the energy of others. The difference in energy is called the crystal field splitting energy. - crystal field theory. crystal field.
- The color, spectra, and magnetic properties of metal-ligand complexes can be explained by modeling the effect of ligands on metal's d orbital energies.
- D-. D-isomer. Compare with L-
 .
. - Prefix used to designate a dextrorotatory
 enantiomer
enantiomer .
. - dextrorotatory.
 dextrorotary. Compare with levorotatory
dextrorotary. Compare with levorotatory .
. - Having the property of rotating plane-polarized light clockwise.
- diazonium salt.

- A diazonium salt is a compound with general form Ar-N
 N+X-, where Ar represents a substituted benzene ring and X- is a halide ion such as chloride. Diazonium salts are unstable and explosive in dry form. They are used to manufacture many different organic compounds, including azo dyes
N+X-, where Ar represents a substituted benzene ring and X- is a halide ion such as chloride. Diazonium salts are unstable and explosive in dry form. They are used to manufacture many different organic compounds, including azo dyes . See also diazotization
. See also diazotization .
. - diazotization.

- Diazotization is a reaction that converts an -NH2 group connected to a phenyl ring to a diazonium salt. For example,
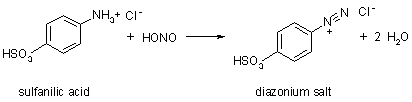
Diazotization reactions are extremely useful in organic synthesis. The nitrous acid provides NO+ which replaces a hydrogen on the -NH3+ group to produce -NH2NO+ and water; a second water is eliminated to produce the -N2+ group.
- dichloromethane.
 (CH2Cl2)
(CH2Cl2) - Dichloromethane (CH2Cl2) is an organic solvent often use to extract organic substances from samples. It is toxic but much less so than chloroform or carbon tetrachloride, which were previously used for this purpose.
- EDTA. ethylenediaminetetracetic acid; versine.
 A polydentate
A polydentate ligand
ligand that tightly complexes certain metal ions. EDTA is used as a blood preservative by complexing free calcium ion (which promotes blood clotting). EDTA's ability to bind to lead ions makes it useful as an antidote for lead poisoning.
that tightly complexes certain metal ions. EDTA is used as a blood preservative by complexing free calcium ion (which promotes blood clotting). EDTA's ability to bind to lead ions makes it useful as an antidote for lead poisoning.- ester.
- An ester is a compound formed from an acid and an alcohol
 . In esters of carboxylic acids
. In esters of carboxylic acids , the -COOH group and the -OH group lose a water and become a -COO- linkage:
, the -COOH group and the -OH group lose a water and become a -COO- linkage: R-COOH + R'-OH = R-COO-R' + H2O
where R and R' represent organic groups. - fatty acid.
- Fatty acids are carboxylic acids
 with long hydrocarbon side chains. Most natural fatty acids have hydrocarbon chains that don't branch; any double bonds occuring in the chain are cis isomers (side chains are attached on the same side of the double bond).
with long hydrocarbon side chains. Most natural fatty acids have hydrocarbon chains that don't branch; any double bonds occuring in the chain are cis isomers (side chains are attached on the same side of the double bond). 
- free radical.
- A free radical is a molecule with an odd number of electrons. Free radicals do not have a completed octet and often undergo vigorous redox reactions. Free radicals produced within cells can react with membranes, enzymes, and genetic material, damaging or even killing the cell. Free radicals have been implicated in a number of degenerative conditions, from natural aging to Alzheimer's disease.
- functional group.
- A substructure that imparts characteristic chemical behaviors to a molecule, for example, a carboxylic acid
 group.
group. - glycerol. (HOCH2CH(OH)CH2OH)
- Glycerol is a small molecule with three alcohol groups. It is a basic building block of fats and oils.


- heterocyclic. heterocycle; heterocyclic ring.
- An organic group or molecule containing rings with at least one noncarbon atom on the ring.
- high spin complex. high-spin complex.
- A metal-ligand complex with the same number of unpaired electrons
 as the uncomplexed metal ion. When a weak ligand
as the uncomplexed metal ion. When a weak ligand complexes the metal ion, the crystal field splitting
complexes the metal ion, the crystal field splitting is small and the electrons can still occupy all of the d orbitals without pairing.
is small and the electrons can still occupy all of the d orbitals without pairing. - homolog. homologue; homologous; homologous series.
- A compound belonging to a series of compounds that differ by a repeating group. For example, propanol (CH3CH2CH2OH), n-butanol (CH3CH2CH2CH2OH), and n-pentanol (CH3CH2CH2CH2CH2OH) are homologs; they belong to a homologous series CH3(CH2)nOH.
- hydrocarbon. Compare with alkane
 , alkene
, alkene , alkyne
, alkyne , and organic
, and organic .
. - Hydrocarbons are organic
 compounds that contain only hydrogen and carbon. The simplest hydrocarbons are the alkanes
compounds that contain only hydrogen and carbon. The simplest hydrocarbons are the alkanes .
. - inductive effect. inductance effect.
- An inductive effect is the polarization of a chemical bond caused by the polarization of an adjacent bond. (Field effects are polarization caused by nonadjacent bonds).
- inorganic chemistry.
- The study of inorganic compounds
 , specifically their structure, reactions, catalysis, and mechanism of action.
, specifically their structure, reactions, catalysis, and mechanism of action. - inorganic compound. inorganic. Compare with organic
 .
. - A compound that does not contain carbon chemically bound to hydrogen. Carbonates, bicarbonates, carbides, and carbon oxides are considered inorganic compounds, even though they contain carbon.
- ketone. (R-CO-R')
- An organic compound
 that contains a carbonyl
that contains a carbonyl group. For example, methyl ethyl ketone is CH3COCH2CH3_ is used in some adhesives.
group. For example, methyl ethyl ketone is CH3COCH2CH3_ is used in some adhesives. - L-. L-isomer. Compare with D-
 .
. - Prefix used to designate a levorotatory
 enantiomer
enantiomer .
. - levorotatory. Compare with dextrorotatory
 .
. - Having the property of rotating plane-polarized light counterclockwise.
- ligand.

- 1. In inorganic chemistry, a molecule or ion that binds to a metal cation to form a complex. 2. In biochemistry, a molecule that binds to a receptor
 , having a biological effect.
, having a biological effect. - low spin complex. low-spin complex. Compare with high spin complex
 .
. - A metal-ligand complex with fewer unpaired electrons
 than the uncomplexed metal ion. When a strong ligand
than the uncomplexed metal ion. When a strong ligand complexes the metal ion, the crystal field splitting
complexes the metal ion, the crystal field splitting is large and some electrons pair rather than occupying the higher energy d orbitals.
is large and some electrons pair rather than occupying the higher energy d orbitals. - methyl. (-CH3)
- A group -CH3, derived from methane. For example, CH3Cl is "methyl chloride" (systematic name: chloromethane); CH3OH is "methyl alcohol" (systematic name: methanol).
- mixed glyceride. Compare with glyceride
 .
. - A diglyceride
 or triglyceride
or triglyceride that contains more than one type of fatty acid
that contains more than one type of fatty acid connected to glycerol
connected to glycerol via an ester
via an ester linkage. Natural oils and fats usually contain several different mixed glycerides.
linkage. Natural oils and fats usually contain several different mixed glycerides. - molecular sieve.
- A material that contains many small cavities interconnected with pores of precisely uniform size. Zeolites
 are an example. Molecular sieves adsorb
are an example. Molecular sieves adsorb molecules that are small enough to pass through their pore systems- especially water. They are often used as drying agents, and to separate large molecules from smaller ones in preparatory work and in exclusion chromatography.
molecules that are small enough to pass through their pore systems- especially water. They are often used as drying agents, and to separate large molecules from smaller ones in preparatory work and in exclusion chromatography. - monodentate.
- A ligand that has only one atom that coordinates directly to the central atom in a complex. For example, ammonia and chloride ion are monodentate ligands of copper in the complexes [Cu(NH3)6]2+ and [CuCl6]2+.
- octane. (C8H18) Compare with alkane
 and hydrocarbon
and hydrocarbon .
. - Flammable liquid compounds found in petroleum and natural gas. There are 18 different octanes- they have different structural formulas but share the molecular formula C8H18. Octane is used as a fuel and as a raw material for building more complex organic molecules. It is the eighth member of the alkane
 series.
series. - optical activity. optically active.
- A substance that is capable of rotating plane-polarized light. Molecules of an optically active substance cannot be superimposed on their own mirror images, just as your left hand cannot be superimposed on your right when both are held palm-down.
- organic. organic compound. Compare with inorganic compound
 .
. - Compounds that contain carbon chemically bound to hydrogen. They often contain other elements (particularly O, N, halogens, or S). Organic compounds were once thought to be produced only by living things. We now know that any organic compound can be synthesized in the laboratory (although this can be extremely difficult in practice!)
- organic chemistry.
- The study of compounds that contain carbon chemically bound to hydrogen, including synthesis, identification, modelling, and reactions of those compounds.
- paraffin. paraffin wax.
- 1. a waxy substance that is a mixture of alkanes
 with chains containing 18 to 36 carbon atoms. 2. An alkane.
with chains containing 18 to 36 carbon atoms. 2. An alkane. - phenol.
- A group or molecule containing a benzene
 ring that has a hydroxyl
ring that has a hydroxyl group substituted for a ring hydrogen.
group substituted for a ring hydrogen. - phenyl. (
 )
) - A molecular group or fragment formed by abstracting or substituting one of the hydrogen atoms attached to a benzene
 ring.
ring. - polydentate. polydentate ligand.
- A ligand
 that has more than one atom that coordinates directly to the central atom in a complex. Polydentate ligands are called chelating agents when two or more coordinating atoms are attached to the same metal ion in a complex. For example, EDTA
that has more than one atom that coordinates directly to the central atom in a complex. Polydentate ligands are called chelating agents when two or more coordinating atoms are attached to the same metal ion in a complex. For example, EDTA or ethylenediaminotetracetic acid is a hexadentate ligand of calcium ion.
or ethylenediaminotetracetic acid is a hexadentate ligand of calcium ion. - polymer.
- A large molecule made by linking smaller molecules ("monomers") together.
- propane. (C3H8) Compare with alkane
 and hydrocarbon
and hydrocarbon .
. - A colorless, odorless, flammable gas, found in petroleum and natural gas. It is used as a fuel and as a raw material for building more complex organic molecules. Propane is the third member of the alkane
 series.
series. - racemic. racemic mixture.
- A mixture of equal parts of the levorotatory
 and dextrorotatory
and dextrorotatory isomers of the the same substance. Racemic mixtures are not optically active
isomers of the the same substance. Racemic mixtures are not optically active .
. - resonance effect. mesomeric effect.
- If electron density at a particular point in a molecule is higher or lower than what you'd expect from a single Lewis structure, and various canonical structures can be drawn to show how electron delocalization will explain the discrepancy, the difference in electron density is called a "resonance effect" or "mesomeric effect".
- strong ligand. strong field ligand. Compare with weak ligand
 .
. - A ligand that causes a large crystal field splitting
 which results in a low-spin complex
which results in a low-spin complex .
. - superoxide. superoxide ion.
- A binary compound containing oxygen in the -½ oxidation state. For example, KO2 is potassium superoxide, an ionic compound containing the superoxide ion, O2-.
- tautomer.
- A structure formed by facile motion of a hydrogen from one site to another within the same molecule.
- thin layer chromatography. (TLC) Compare with chromatography
 .
. - A technique for separating components in a mixture on the basis of their differing polarities. A spot of sample is placed on a flat sheet coated with silica and then carried along by a solvent that soaks the sheet. Different components will move different distances over the surface. TLC is a useful screening technique in clinical chemistry; for example, it can be used to detect the presence of drugs in urine.
- triglyceride.
- A triglyceride is an ester
 of glycerol
of glycerol and three fatty acids
and three fatty acids . Most animal fats are composed primarily of triglycerides. In the structures below, the fatty acids attached to the glycerol are represented by 'R'. The fatty acids can be the same or different.
. Most animal fats are composed primarily of triglycerides. In the structures below, the fatty acids attached to the glycerol are represented by 'R'. The fatty acids can be the same or different. 

- unsaturated compound.
- An organic compound with molecules containing one or more double bonds.
- water gas. blue gas; synthesis gas.
- A fuel gas used in industrial synthesis of organic chemicals, and in welding, glassmaking, and other high-temperature industrial applications. Water gas made by passing steam over a bed of hot coal or coke. It consists mainly of of carbon monoxide (CO) and hydrogen (H2), contaminated with small amounts of CO2, N2, CH4, and O2.
- wax.
- An ester
 formed from long-chain fatty acids
formed from long-chain fatty acids and alcohols
and alcohols that is usually solid at room temperature.
that is usually solid at room temperature. - weak ligand. weak field ligand. Compare with strong field ligand
 .
. - A ligand that causes a small crystal field splitting
 which results in a high-spin complex
which results in a high-spin complex .
. - -yl.
- A suffix indicating a molecular fragment or group; e. g. a methyl group (-CH3) derived from methane (CH4).
- zwitterion.
- A particle that contains both positively charged and negatively charged groups. For example, amino acids
 (NH2-CHR-COOH) can form zwitterions (+NH3-CHR-COO-)
(NH2-CHR-COOH) can form zwitterions (+NH3-CHR-COO-)
General Chemistry Online! Introduction to organic chemistry
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/organic/glossary.shtml