
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
| Redox reactions |
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Redox reactions Redox reactions | Print | Comment |
| Previous Question | Next Question |
What is an activity series, and how is it used?
-
-
An activity series is a list of substances ranked in order of relative reactivity. For example,
magnesium metal can knock hydrogen ions out of solution, so it is considered more reactive than elemental
hydrogen:
Mg(s) + 2 H+(aq)
Zinc can also displace hydrogen ions from solution: H2(g) + Mg2+(aq)
H2(g) + Mg2+(aq)
Zn(s) + 2 H+(aq)
so zinc is also more active than hydrogen. But magnesium metal can remove zinc ions from solution: H2(g) + Zn2+(aq)
H2(g) + Zn2+(aq)
Mg(s) + Zn2+(aq)
The reaction goes nearly to completion [Note 1]. Magnesium is more active than zinc, and the activity series including these elements would be Mg > Zn > H. The following activity series built up in a similar way. The most active metals are at the top of the table; the least active are at the bottom. Each metal is able to displace the elements below it from solution (or, using the language of electrochemistry, each metal can reduce the cations of metals below it to their elemental forms). Zn(s) + Mg2+(aq)
Zn(s) + Mg2+(aq)
The metal activity series. Most active (most strongly reducing) metals appear on top, and least active metals appear on the bottom.displace H2 from water, steam, or acids Li 2 Li(s) + 2 H2O( 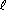 )
)  2 LiOH(aq) + H2(g)
2 LiOH(aq) + H2(g)K 2 K(s) + 2 H2O( 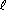 )
)  2 KOH(aq) + H2(g)
2 KOH(aq) + H2(g)Ca Ca(s) + 2 H2O( 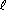 )
)  Ca(OH)2(s) + H2(g)
Ca(OH)2(s) + H2(g)Na 2 Na(s) + 2 H2O( 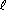 )
)  2 NaOH(aq) + H2(g)
2 NaOH(aq) + H2(g)displace H2 from steam or acids Mg Mg(s) + 2 H2O(g)  Mg(OH)2(s) + H2(g)
Mg(OH)2(s) + H2(g)Al 2 Al(s) + 6 H2O(g)  2 Al(OH)3(s) + 3 H2(g)
2 Al(OH)3(s) + 3 H2(g)Mn Mn(s) + 2 H2O(g)  Mn(OH)2(s) + H2(g)
Mn(OH)2(s) + H2(g)Zn Zn(s) + 2 H2O(g)  Zn(OH)2(s) + H2(g)
Zn(OH)2(s) + H2(g)Fe Fe(s) + 2 H2O(g)  Fe(OH)2(s) + H2(g)
Fe(OH)2(s) + H2(g)displace H2 from acids only Ni Ni(s) + 2 H+(aq)  Ni2+(aq) + H2(g)
Ni2+(aq) + H2(g)Sn Sn(s) + 2 H+(aq)  Sn2+(aq) + H2(g)
Sn2+(aq) + H2(g)Pb Pb(s) + 2 H+(aq)  Pb2+(aq) + H2(g)
Pb2+(aq) + H2(g)H2 can't displace H2 Cu Ag Pt Au The activity series is a useful guide for predicting the products of metal displacement reactions. For example, placing a strip of zinc metal in a copper(II) sulfate solution will produce metallic copper and zinc sulfate, since zinc is above copper on the series. A strip of copper placed into a zinc sulfate solution will not produce an appreciable reaction, because copper is below zinc on the series and can't displace zinc ions from solution.
The series works well as long as the reactions being predicted occur at room temperature and in aqueous solution. It isn't difficult to find reactions that are at odds with the metal and nonmetal activity series under other conditions. There are other complications too. For example, aluminum would be expected to displace hydrogen from steam, but in fact it won't unless the aluminum oxide film on its surface is scrubbed off. Copper can't displace hydrogen from acids, but it does react with acids like nitric and sulfuric because they can act as oxidizing agents.
It might be expected that metals with lower ionization energies and lower electronegativities would be more active, since they would be expected to more easily lose electrons in a displacement reaction. But while ionization energy and electronegativity do affect a metal's ranking in the series, other factors have a strong and complex influence on relative activity [Note 2], obscuring the relationship.
Activity series can be devised for nonmetals as well. Since nonmetallic elements tend to accept electrons in redox reactions, the nonmetal activity series is arranged so that the most powerful oxidizing agents are considered most active (whereas in the metal series, the most powerful reducing agents are the most active):
For example, the series predicts that Cl2 will displace Br- and I- from solution, because Cl2 appears above Br2 and I2:The nonmetal activity series. Most active (most strongly oxidizing) nonmetals appear on top, and least active nonmetals appear on the bottom.F2 strongest oxidizing agent Cl2 O2 Br2 I2 S red P weakest oxidizing agent Cl2(g) + 2 Br-(aq)
 2 Cl-(aq) + Br2(
2 Cl-(aq) + Br2(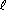 )
)
Cl2(g) + 2 I-(aq) 2 Cl-(aq) + I2(s)
2 Cl-(aq) + I2(s)
Br2(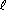 ) + 2 Cl-(aq)
) + 2 Cl-(aq)  no reaction
no reaction
I2(s) + 2 Cl-(aq) no reaction
no reaction
Notes
- The reverse reaction does occur- a very tiny amount of zinc placed in a solution of magnesium ions will dissolve. The reaction is actually an equilibrium, but it lies far to the right. Back
- Ionization energies and electronegativies are derived for the gas phase- while the activity series
applies to solutions, under standard conditions. Enthalpies of hydration and sublimation for the metal also must be considered
in rationalizing its place in the activity series.
For a more detailed discussion of these points, see Dr. R. Peters' AUFBAU1 notes.
Also, a metal's rank on the activity series is determined by the free energy change it undergoes when it transfers electrons in a redox reaction. The free energy change includes contributions from the entropy change associated with the process as well as from the energy required or released. Even when the ionization energies, sublimation energies, and hydration energies of two metals are very similar, the entropy contribution can make their activities quite different. Back
Resources
-
The Fall of the Electron
(Stephen Lower, Simon Fraser U.) 
The activity series is a very helpful tool for explaining and predicting the direction of redox reactions. The series ranks metals by "oxidation potential". Top-ranked metals displace the ions of metals lower in the series from solution. A free-energy based series is used in this tutorial to show how and why active metals displace less active ones from solution.
http://www.sfu.ca/chemcai/AQCHEM/FallElect.html (01/19/99)
General Chemistry Online! What is an activity series, and how is it used?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/redox/faq/activity-series.shtml