
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
| Energy & change |
Electrons in atoms
The periodic table
Chemical bonds
Solids
Liquids
Solutions
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Energy and chemical change Energy and chemical change | Print | Comment |
| Previous Question | Next Question |
Why can we measure enthalpy changes, but not absolute enthalpies?
- What would be considered the simplest explanation for high school students regarding why enthalpy cannot be measured, although changes in enthalpy can be?
Thomas Moore -
At this level, just explain that "enthalpy change" is
a synonym for "heat at constant pressure". For example, if an object absorbs 100 J of heat at 1 atm, you can say "the enthalpy of this object increased by 100 J." But you really know nothing about the value of the enthalpy before or after the heat was absorbed.
A metaphor using a change in a more familiar state property that is impractical or impossible to measure absolutely is very helpful. For example:
- You buy groceries worth $42.00. You can't say what your net financial worth is at that instant, down to the penny; but you can tell they're $42.00 less than before you bought groceries. It doesn't really matter what your net worth is; nothing interesting happens unless you spend money, or earn money.
- You climb to the top of a building that is 203 feet high. You don't know how far away the center of the earth is, but you know it's 203 feet further away than it was before you climbed the building.
- You dump a liter of water into the ocean. You don't know what the volume of the ocean is, but you know it's a liter more than it was before you came along.
Note that you're free to define a reference point, though, and measure enthalpies relative to that reference point (as we do with heats of formation).
At a more advanced level, enthalpy is defined in terms of internal energy: H = U + PV. The definition was made solely to provide a convenient state function for constant pressure calorimetry (so that
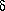 H = qP).
H = qP).
General Chemistry Online! Why can we measure enthalpy changes, but not absolute enthalpies?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/thermo/faq/enthalpy-changes.shtml