
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
| Chemical bonds |
Liquids
Solutions
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Chemical bonds Chemical bonds | Print | Comment |
| Previous Question | Next Question |
How do I explain bonding in O2 using hybridization?
-
-
Let's use O2 as a specific example. It's known experimentally that the length and strength of the bond between the oxygens suggests a double bond. The valence bond theory views multiple bonds as overlaps between orbitals that lie off the bond axis, on top of an overlap that occurs on the bond axis. The overlap on the bond axis is called a sigma bond. The overlaps off the bond axis are called pi bonds.
- Draw a Lewis structure for the molecule. For O2, it's
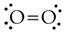
- Count how many electron 'regions' you have around each atom in the molecules. A region is either a bond or a lone pair. Multiple bonds count as just one region. In O2, there are 3 regions around each atom.
- Chose a hybridization scheme for each atom that is consistent with the number of regions around that atom:
Regions Possible
HybridizationShape 2 sp linear (w/ 2 unhybridized p's) 3 sp2 triangular planar (w/ 1 unhybridized p) 4 sp3 tetrahedral 5 dsp3 triangular bipyramidal 6 d2sp3 octahedral - Sketch the hybrid orbitals for each atom. For O2,

- Sketch the valence orbitals that are unhybridized. The O's are sp2 hybridized, so there is one unhybridized p orbital at right angles to the hybridized orbitals. The p orbital is drawn in blue:

- Overlap orbitals to get the right number of bonds. The oxygens have a hybridized sp2 orbital and an unhybridized p that can overlap to give the double bond. Notice that the hybridized orbital overlap is a sigma bond, because it is along the bond axis; the unhybridized p's overlap side to side to give a pi bond, which is off the bond axis.

- Draw a Lewis structure for the molecule. For O2, it's
General Chemistry Online! How do I explain bonding in O_2_ using hybridization?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/bonds/faq/explaining-o2-with-vb.shtml