
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
 Glossary
GlossaryGlossary: O
- octane. (C8H18) Compare with alkane
 and hydrocarbon
and hydrocarbon .
. - Flammable liquid compounds found in petroleum and natural gas. There are 18 different octanes- they have different structural formulas but share the molecular formula C8H18. Octane is used as a fuel and as a raw material for building more complex organic molecules. It is the eighth member of the alkane
 series.
series. - octet.
- A set of eight valence electrons
 .
. - octet rule.
- A guideline for building Lewis structures
 that states that atoms tend to gain, lose, or share valence electrons
that states that atoms tend to gain, lose, or share valence electrons with other atoms in a molecule until they hold or share eight valence electrons. The octet rule almost always holds for carbon, nitrogen, oxygen, and fluorine; it is regularly violated for other elements.
with other atoms in a molecule until they hold or share eight valence electrons. The octet rule almost always holds for carbon, nitrogen, oxygen, and fluorine; it is regularly violated for other elements. - ohm.
- The SI
 unit of electrical resistance
unit of electrical resistance , equal the resistance between two points when a constant voltage produces an electric current
, equal the resistance between two points when a constant voltage produces an electric current of 1 ampere
of 1 ampere .
. - ohmmeter.
- An instrument for measuring electrical resistance
 .
.
- oligosaccharide. Compare with monsaccharide
 and polysaccharide
and polysaccharide .
. - A carbohydrate
 that consists of only a few linked monosaccharide
that consists of only a few linked monosaccharide units.
units. - optical activity. optically active.
- A substance that is capable of rotating plane-polarized light. Molecules of an optically active substance cannot be superimposed on their own mirror images, just as your left hand cannot be superimposed on your right when both are held palm-down.
- orbital.
- A wavefunction
 that describes what an electron with a given energy is doing inside an atom or molecule.
that describes what an electron with a given energy is doing inside an atom or molecule. - order. order of reaction; reaction order.
- The order of a reaction is the sum of concentration exponents in the rate law for the reaction. For example, a reaction with rate law d[C]/dt = k[A]2[B] would be a third order reaction. Noninteger orders are possible.
- organic. organic compound. Compare with inorganic compound
 .
. - Compounds that contain carbon chemically bound to hydrogen. They often contain other elements (particularly O, N, halogens, or S). Organic compounds were once thought to be produced only by living things. We now know that any organic compound can be synthesized in the laboratory (although this can be extremely difficult in practice!)
- organic chemistry.
- The study of compounds that contain carbon chemically bound to hydrogen, including synthesis, identification, modelling, and reactions of those compounds.
- organochromic indicators.
- Colored organic compounds that change color when they chelate
 different metals. Organochromic indicators are used to determine the endpoint
different metals. Organochromic indicators are used to determine the endpoint in complexometric titrations
in complexometric titrations . Examples of organochromic indicators are are Eriochrom Black T, calmagite, and Eriochrom Cyanine R.
. Examples of organochromic indicators are are Eriochrom Black T, calmagite, and Eriochrom Cyanine R. - osmometry. Compare with osmosis
 .
. - Determination of the average molecular weight of a dissolved substance from measurements of osmotic pressure
 .
. - osmosis. Compare with reverse osmosis
 .
. - Passage of solvent molecules from a dilute solution through a semipermeable membrane
 to a more concentrated solution.
to a more concentrated solution. - osmotic pressure.
- Pressure which must be applied to a solution to prevent water from flowing in via a semipermeable membrane
 .
. - oxidation. oxidize; oxidizing; oxidized. Compare with reduction
 .
. - Oxidation is the loss of one or more electrons by an atom, molecule, or ion. Oxidation is accompanied by an increase in oxidation number on the atoms, molecules, or ions that lose electrons.
- oxidation half reaction. oxidation half-reaction. Compare with reduction half reaction
 .
. - That part of a redox reaction
 that involves loss of electrons. In the oxidation half reaction, the oxidation number
that involves loss of electrons. In the oxidation half reaction, the oxidation number of one or more atoms within the reactants is increased.
of one or more atoms within the reactants is increased.
- oxidizing agent. oxidant; oxidizer. Compare with reducing agent
 .
. - A reactant that removing electrons from other reactants in a chemical reaction. Oxidizing agents cause other substances to be oxidized in chemical reactions while they themselves are reduced. For example, nitrate ion is an oxidizing agent in the following reaction:
Cu(s) + 4 H+(aq) + 2 NO3-(aq)
Copper gets oxidized (its oxidation number goes from 0 to +2) while the nitrogen gets reduced (from +5 in nitrate to +4 in nitrogen dioxide). Cu2+(aq) + 2 H2O(
Cu2+(aq) + 2 H2O(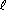 ) + 2 NO2(g)
) + 2 NO2(g) - oxidation number. oxidation state; positive valence.
- A convention for representing a charge of an atom embedded within a compound, if the compound were purely ionic. For example, H2O is a covalent compound; if it were ionic, the hydrogens would be H+ (oxidation number +1) and the oxygen would be O2- (oxidation number -2). Oxidation number rises for at least one atom in a compound that is oxidized
 ; oxidation number becomes smaller if the compound is reduced
; oxidation number becomes smaller if the compound is reduced .
. - oxide.
- A binary compound
 that contains oxygen in the -2 oxidation state
that contains oxygen in the -2 oxidation state .
.
- oxygen. (O)
- Element 8, atomic weight 15.9994, a colorless, odorless gas that makes up about 1/5 of the earth's atmosphere and (in combined form) 8/9ths of earth's oceans and almost half of the earth's crust. The name is derived from the French oxygène, which means "acid generating".

General Chemistry Online! Glossary: O
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/glossary/o.shtml