
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
The quantum theory
Electrons in atoms
The periodic table
Chemical bonds
Solids
| Liquids |
Acids & bases
Redox reactions
Reaction rates
Organic chemistry
Everyday chemistry
Inorganic chemistry
Environmental chemistry
Laboratory
History of chemistry
Miscellaneous
Home  FAQ FAQ  Liquids Liquids | Print | Comment |
| Previous Question |
What factors affect the size of fluid drops exiting a dropper or a burette?
-
-
The droplets themselves are the result of a phenomenon called surface tension. Consider this crude molecular snapshot of a droplet:
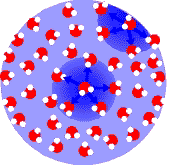 Water molecules attract each other rather strongly due to their polarity. Molecules deep within the drop are pulled every which way by their neighbors. Since they are surrounded more or less symmetrically, they experience little or no net force.
Water molecules attract each other rather strongly due to their polarity. Molecules deep within the drop are pulled every which way by their neighbors. Since they are surrounded more or less symmetrically, they experience little or no net force.
Molecules on the droplet surface don't have neighbors on one side. There is a strong net force from the tug of the water molecules below them, which pulls them towards the center of the droplet.
Surface tension is the work you have to do to increase the area of the drop by a unit amount. Since work has units of force times length, surface tension has units of force per unit length.
A small amount of liquid in a very thin dropper won't run out unless you blow it out- it's held in the tube by the surface tension of the drop on the tip, which exerts an upward force. If there is enough liquid in the tube, though, there will come a point where the weight of the liquid overwhelms upward force, and the drop will fall.
If surface tension is force per unit length, you can crudely predict the weight of that droplet by multiplying the circumference of the dropper tip by the liquid's surface tension. For example,
- a 1 mm diameter dropper has a circumference of pi times the diameter, or 0.314 cm.
- water has a surface tension of about 72.9 g/s2. These are units of force (g cm/s2) per length (cm).
- The upward force on due to surface tension is then about 0.314 cm × (72.9 g/s2) = 23 g cm/s2.
- The weight required to overcome the upward force will also be 23 g cm/s. That corresponds to a droplet mass of about 0.023 g.
General Chemistry Online! What factors affect the size of fluid drops exiting a dropper or a burette?
Copyright © 1997-2010 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 02/23/18.URL: http://antoine.frostburg.edu/chem/senese/101/liquids/faq/tates-law.shtml