
Home
Common Compounds
Exam Guide
FAQ
Features
Glossary
Construction Kits
Companion Notes
Just Ask Antoine!
Simulations
Slide Index
Toolbox
Tutorial Index
Companion Notes
Introduction
Measurement
Matter
Atoms & ions
Compounds
Chemical change
The mole
Gases
Energy & change
| The quantum theory |
The periodic table
Home  Companion Notes Companion Notes | Print | Comment |

|
Learning objectives
- Relate wavelength
 , frequency
, frequency , and velocity of waves.
, and velocity of waves.
- Explain how electromagnetic radiation
 is produced by an oscillating charge.
is produced by an oscillating charge.
- Explain how electromagnetic radiation carries energy from a transmitter to a receiver.
- Describe the collapsing atom paradox.
- List wave behaviors, and distinguish them from particle behaviors.
- Cite experimental evidence that implies that electromagnetic radiation can display both wave and particle behaviors.
- Cite experimental evidence that implies that electrons display both wave and particle behaviors.
- Connect particle and wave properties of matter using de Broglie's hypothesis.
- Explain what a standing wave is.
- Compare a wave on a wire, a particle on a wire, and an electron on a wire.
- Show how de Broglie's hypothesis implies the existence of quantized energy states for standing electron waves.
- Show how quantum numbers arise for standing electron waves.
- State Heisenberg's uncertainty principle, and explain why it resolves the collapsing atom paradox.
Lecture outline
Electrons behave like particles in some experiments, and like waves in others. The electron's 'wave/particle duality' has no real analogy in the everyday world. The quantum theory that describes the behavior of electrons is a cornerstone in modern chemistry. Quantum theory can be used to explain why atoms are stable, why things have the color they do, why the periodic table has the structure it does, why chemical bonds form, and why different elements combine in different ratios with each other.Light and electrons both behave quantum mechanically. To understand the experimental basis for the quantum theory, we have to begin our discussion with light.
Waves
- Waves are an oscillation that moves outward from a disturbance (ripples moving away from a pebble dropped into a pond)
Properties of waves
property definition symbol SI units velocity distance traveled per second c m/s amplitude peak height above midline A varies with type of wave wavelength peak-to-peak distance 
m frequency number of peaks passing by per second 
s-1 (called Hertz  )
) - relationship between frequency and wavelength
- distance per cycle × cycles per second = distance per second

 = c
= c
- examples
- The speed of sound in air is 330 m/s. Humans can hear sounds with wavelengths between 17 m and 17 mm. What is the highest sound frequency that is audible?
- distance per cycle × cycles per second = distance per second
- interference

- constructive interference: amplitudes add
- peaks, troughs of interfering waves occur in the same positions (waves are in phase )
- destructive interference: amplitudes cancel
- peaks of one wave are in same position as troughs of the other (waves are out of phase )
- constructive interference: amplitudes add
- diffraction




Waves can bend
around small obstacles……and fan out
from pinholes.particles effuse  from pinholes.
from pinholes.- a wave can't bend around obstacles much larger than its wavelength
- what does this imply about the wavelength of sound waves? radio waves? visible light?
- a wave can't bend around obstacles much larger than its wavelength
- waves are delocalized (spread out in space)
Three ways to tell a wave from a particle
wave behavior particle behavior waves interfere particles collide waves diffract particles effuse waves are delocalized particles are localized
Is light a stream of particles or a wave?
- Thomas Young, 1801
- pass light through two tiny adjacent slits
- if light were particles:
- target would be brightest where light passing through the slits overlapped
- target would darken steadily moving away from the overlap region
- this was not observed!
- a pattern of light and dark stripes was observed instead
- Young explained the stripes as a combination of diffraction and interference
- these interference fringes are a sure sign of wave behavior

- White areas are peak-peak or trough-trough overlaps (constructive interference
 )
) - black areas are peak-trough overlaps (destructive interference
 ).
).
- width of interference bands suggested the wavelength of visible light was less than a millionth of a meter!
- If light is a wave, what is oscillating?
- light can move through a vacuum, so it isn't an oscillation of atoms as sound and water waves are.
Force Fields
- force field: a region where forces act on an object; strength of forces vary with position
- gravitational fields
- larger mass at center of field = stronger forces
- larger distance from center of field = weaker forces
- electric fields
- opposite charges attract each other, but like charges repel each other
- larger charge at center of field = stronger forces
- larger distance from center of field = weaker forces
- magnetic fields
- can be produced by moving charges (electromagnets)
- a moving magnetic field can produce an electric field (electric generator)
Electromagnetic radiation
- James Clerk Maxwell ca. 1855
- changes in electric and magnetic fields are always coupled: electromagnetism
- making e/m waves with a vibrating charge
- both electric and magnetic fields oscillate
- oscillations are at right angles

- electric oscillation produces magnetic oscillation, which produces another electric oscillation, …and on and on
- vibrating charge creates a ripple in the electromagnetic field
- The speed of electromagnetic radiation was computed to be around 3×108 m/s
- The same speed had been determined experimentally for light!
- hypothesis: light is a form of electromagnetic radiation (Maxwell, 1862)
 |
 |
The electromagnetic spectrum
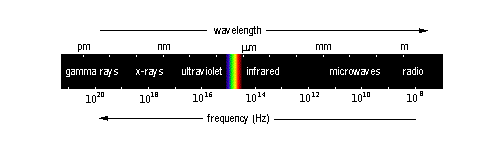
| region of spectrum | wavelengths | typical source |
| radio | more than 30 cm | radio, television |
| microwave | 3 mm to 30 cm | radar, microwave oven |
| infrared | 750 nm to 3 mm | hot objects |
| visible | 400 nm to 750 nm | very hot objects |
| ultraviolet | 20-400 nm | sun; black lights |
| x-rays | 3 pm to 20 nm | cathode ray tubes |
| gamma rays | less than 3 pm |
Energy of electromagnetic radiation
- radiation carries energy through space
- work is done on charges in the e/m field
- transmitter loses energy; reciever gains energy
- for classical waves:
- higher amplitude means higher energy per peak
- amplitude squared determines the intensity or brightness of light
- therefore, brighter light should carry more energy per peak than dimmer light

- an experiment to measure the energy carried by an electromagnetic wave
- photoelectric effect: shining light on alkali metals knocks electrons out of metal
- strategy: measure kinetic energy of ejected electrons; then measure light energy per ejected electron.
- surprise:
- brightness has NO EFFECT on the kinetic energy per ejected electron
- brighter light ejects MORE electrons.
- surprise #2:
- red light can't eject any electrons, but blue light can!
- below a threshold frequency (
 0), there are no ejected electrons!
0), there are no ejected electrons!
 0 is a property of the metal being used
0 is a property of the metal being used
- below a threshold frequency (
- red light can't eject any electrons, but blue light can!
- Albert Einstein's interpretion of the photoelectric effect (Nobel Prize, 1921)
- maybe light is like a stream of massless particles (call them photons)
- brighter light has more photons, but bluer light has higher energy photons
- frequency-to-energy conversion factor is h (Planck's constant, 6.626×10-34 J/Hz)

- examples
- What is the energy of a photon of red light with wavelength 700 nm?
- What is the wavelength of a photon which has an energy of 1×10-18J?
- Shining light of 400 nm on a metal causes electrons with a kinetic energy of 5×1019 J to be ejected. What is the minimum energy required to eject an electron from the metal?
 .
.
| energy per photon |
= | energy to pull one electron out of metal |
+ | kinetic energy per ejected electron |
| Ephoton | = | h 0 0 |
+ | h( - - 0) 0)
|
| Ephoton | = | h |
The collapsing atom paradox
- what's the electron doing in an atom?
- electrons within the atom can't be stationary:
- positively charged nucleus will attract the negatively charged electron
- electron will accelerate towards the nucleus
- if electrons within the atom move,
- moving charges emit electromagnetic radiation
- emission will cause electrons to lose energy and spiral into the nucleus
- the atom will collapse!
- why don't atoms collapse?
- classical physics has no answer!
- key: electrons have wave/particle duality
Electrons as Waves
- the de Broglie hypothesis (Nobel Prize, 1929)
- connect wave and particle nature of matter using a relationship that applies to photons:
 = h/p
= h/p
 of the particle (p = mass times velocity).
of the particle (p = mass times velocity).
- examples
Compute the de Broglie wavelength of a tennis ball (m = 0.1 kg) and an electron (m = 9.1 x 10-31 kg), if both are moving at 1000 m/s.
- connect wave and particle nature of matter using a relationship that applies to photons:
- experimental evidence of electron wave/particle duality
- electron diffraction
- C. J. Davisson and G. P. Thomson observed interference fringes when electron beams hit crystal surfaces and thin metal films (Nobel Prize, 1937)

Electron diffraction pattern collected from crystalline silicon
Semiconductor Surface Physics Group
Queens University
- C. J. Davisson and G. P. Thomson observed interference fringes when electron beams hit crystal surfaces and thin metal films (Nobel Prize, 1937)
- electrons passing one at a time through a double slit. Each spot shows an electron impact on the detector.

100 electrons 
3000 electrons 
70000 electrons
…interference fringes!!! - applications
- LEED surface analysis
- electron microscopy
- electron diffraction
Bound electrons have quantized energies
- model I: bead on a wire
- kinetic energy of bead can have any value, because velocity can have any value
- bead can be stationary
- bead is equally likely to be found anywhere on the wire
- exact position and velocity of the bead can be known simultaneously
- model II: wave on a wire
- there must be a whole number of peaks and troughs on the wire:
n ( /2) = L, where:
/2) = L, where:
- n is an integer (1, 2, 3, ... )
-
 is the wavelength
is the wavelength
- L is the length of the wire
- standing waves have quantized wavelengths
- there must be a whole number of peaks and troughs on the wire:
- model III: electron on a wire
- unite wave and bead models using the de Broglie relation:
- unite wave and bead models using the de Broglie relation:
| particle kinetic energy: |  |
| de Broglie wave/particle relation: | |
| standing wave allowed wavelengths: |
- The fact that E depends on an integer n means that only certain energy states are allowed
- the integer n labels each state; n is a quantum number
- Notice that n can't be zero, so the lowest energy state is not zero: the electron is never at rest!
- peaks and troughs correspond to buildup of negative charge- electron is not equally likely to be found anywhere on the wire.
- electrons behave like waves
- bound waves have restricted wavelengths
- therefore, electrons bound in atoms and molecules have restricted energies
The uncertainty principle
- Quantum theory puts a limit on the precision of measurements
- Werner Heisenberg's uncertainty principle (Nobel Prize, 1932)
- You can never know both the exact position and the exact momentum of a particle.
- Mathematical statement:
 x
x (mv)
(mv)  h/2
h/2
where
 x
xis the error in a measurement of the particle's position, x m is the particle's mass, v is its velocity,  (mv)
(mv)is the error in a measurement of the particle's momentum, mv h is Planck's constant (6.626 × 10-34 Js).
- The uncertainty principle says that the act of measurement changes what you're trying to measure
- You can bounce a photon off a brick wall, and the wall doesn't change much...
- ...but bouncing a photon off an electron to measure its position will change its momentum
- Why atoms don't collapse
- The uncertainty in position is also the smallest space a particle can possibly be restricted to
- The position of an electron can't possible be known to better than ± 4 × 10-13 m by the uncertainty principle
- … so an electron can't be confined within the nucleus
newsletter describing updates,
new features, and changes
on this site.
General Chemistry Online! The quantum theory
Copyright © 1997-2005 by Fred Senese
Comments & questions to fsenese@frostburg.edu
Last Revised 12/14/21.URL: http://antoine.frostburg.edu/chem/senese/101/quantum/index.shtml